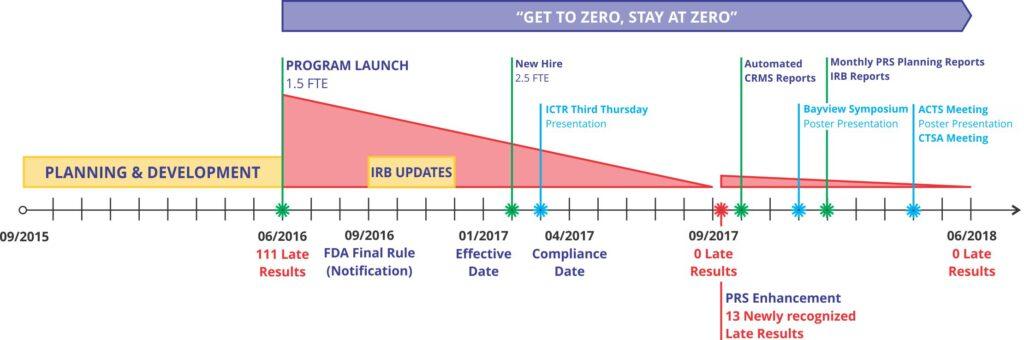
The ClinicalTrials.gov (CT.gov) Program assists research teams with the registration and reporting requirements for clinical trials to avoid federal penalties.
The ClinicalTrials.gov program provides:
- The ethical, scientific and legal reasons for clinical trials registration and reporting
- Information regarding which trials are required to be registered and the timelines
- Tips, tricks and helpful content to improve the process
- Up-to-date information on institutional and federal policies
- Direct effort upon request (billable/hr.)
ClinicalTrials.gov Administrators
Protocol Registration and Results System (PRS) Administrators have access to all records within an institutional account. They create and modify user accounts, and assist users with all aspects of registration, updating and results reporting. If you have any questions please use the information below to contact the appropriate Administrator. We are here to help.
- JohnsHopkinsU (SOM, SON) – [email protected]
- Oncology (SKCCC) – [email protected]
- BSPH – [email protected]
- Kennedy Krieger Institute – Eun Sol Jung [email protected]
- All Children’s Hospital – [email protected]
Publications
Addressing the quality of submissions to ClinicalTrials.gov for registration and results posting: The use of a checklist.
Tetteh, O., Nuamah, P., Keyes, A. Addressing the quality of submissions to ClinicalTrials.gov for registration and results posting: The use of a checklist. Society of Clinical Trials. Published online August 5, 2020. https://doi.org/10.1177/1740774520942746
Time from Submission of Johns Hopkins University Trial Results to Posting on ClinicalTrials.gov
Keyes A, Mayo-Wilson E, Atri N, et al. Time From Submission of Johns Hopkins University Trial Results to Posting on ClinicalTrials.gov. JAMA Intern Med. 2020;180(2):317–319. doi:10.1001/jamainternmed.2019.4710 https://jamanetwork.com/journals/jamainternalmedicine/article-abstract/2753423
BioMed Central Publication (Supported by the ICTR detailing survey results of academic organizations)
Mayo-Wilson E, Heyward J, Keyes A, Reynolds J, White S, Atri N, Alexander C, Omar A, Ford DE, on behalf of the National Clinical Trials Registration and Results Reporting Taskforce Survey Subcommittee. Clinical trial registration and reporting: A survey of academic organizations in the United States. BMC Medicine. 2018;16:60. https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-018-1042-6.
Presentations
- Third Thursdays with the ICTR, ClinicalTrials.gov Program, Meeting the Demand for Transparency through ClinicalTrials.gov Compliance (June 20, 2019) Video | Slides
- ClinicalTrials.gov Registration & Reporting Guidelines and Best Practices (September 17, 2018) Video
- Helping Research Teams Address New NIH Application Requirements Around Clinical Trials (March 5, 2018) Video | Slides
- Third Thursdays with the ICTR, Incorporating the Final Rule (March 16, 2017) Video | Slides
Handouts
Guide for Principal Investigators
Using Admin Changes in IRB_20210409
Work Instruction- Anticipated Start Date
Work Instruction- Anticipated Study Completion Date
Work Instruction- Anticipated Primary Completion Date
Work Instruction- Anticipated Primary Study Completion Date
Work Instruction- Anticipated Verification Date
FAQs
To school of medicine faculty and staff (January 9, 2018)
Dear Colleagues:
We are writing to share a new resource to assist investigators who plan to leave The Johns Hopkins University. The departing investigator checklist is a comprehensive list of the steps that need to be taken to ensure appropriate handling of physical research resources such as lab equipment and material, and smooth and orderly transition of the administrative and fiscal aspects of research, such as grants and contracts, IRB protocols, INDs and IDEs and IACUC protocols.
Winding down or transferring research labs and programs can be complex, so we urge you to review the checklist and begin these procedures as early as possible, but at least two months before your anticipated departure date. Please contact your department director and the school of medicine’s Office of Research Administration with any questions.
Sincerely,
Daniel Ford, M.D.
Vice Dean for Clinical Investigation
Johns Hopkins University School of Medicine
Antony Rosen, M.B. Ch.B., B.Sc. (Hons)
Vice Dean for Research
Johns Hopkins University School of Medicine
Guidance for Investigators Related to their Research upon Leaving Johns Hopkins University
- If the departing investigator is the PI, Record Owner, or otherwise named on any record registered under a Johns Hopkins entity on ClinicalTrials.gov, the departing investigator must contact the Johns Hopkins ClinicalTrial.gov program at [email protected] to arrange for any necessary record transfer.
Clinical trial registration information will be requested with the initial IRB application. If the trial has not been registered at the time of initial IRB approval, the investigator will mark the National Clinical Trials (NCT) number as pending.
It is the responsibility of the PI to update the IRB application with the NCT number in accordance with the following timeline:
- ICMJE requires trial registry at or before first patient enrollment as a condition for publication
- The Food and Drug Administration Amendments Act (FDAAA) requires that the Responsible Party for an Applicable Clinical Trial must submit required clinical trial information through the Protocol Registration and Reporting System (PRS) no later than 21 days after enrollment of the first participant.
A “Change in Research” is no longer required to update the ClinicalTrials.gov National Clinical Trials (NCT) number within your Institutional Review Board (IRB) application. Simply utilize the “Admin Changes” function, which can be found on the left hand margin of your study “Application Workspace.” Promptly and accurately updating eIRB is required whether the record was registered by the study team (through a Johns Hopkins account) or by the Sponsor.
Using the Admin Changes Function within the IRB
You may also need to update the NCT in CRMS.
In order for us to create a user account, you must first submit a service request. Please complete all fields, your request will not be accepted if you do not provide complete information.
- Commitment to research participants
- Scientific validity/transparency
- Ethical standards
- Responsible stewardship of federal funds
- Required by law (FDAAA)
- Required for any clinical trial funded in full or in part by NIH
- Required by NCI
- Required for journal publication (ICMJE)
- Required by many Foundations (e.g., Bill and Melinda Gates Foundation, Wellcome Trust)
- Required for many MRI studies (e.g., functional, breath-hold, respiratory-challenge, and gadolinium)
- Required for CMS
- Required by WHO
The Responsible Party (RP) for a clinical trial must register the trial and submit results information. An RP can be:
- The Sponsor of the clinical trial (as defined in section 21 CFR 50.3) who initiates the study. The Johns Hopkins PI should consult with commercial sponsors to assure that posting of a trial is in accord with terms of the study contract.
- The Principal Investigator (PI) of such clinical trial, assuming;
- the PI is responsible for conducting the trial,
- has access to and control over the data from the clinical trial,
- has the right to publish the results of the trial, and
- has the ability to meet all of FDAAA’s requirements for the submission of clinical trial information.
- The Sponsor-Investigator (the individual who both initiates and conducts the study)
Per updated Policy 103.25 Organization Policy on Registration of Clinical Trials Johns Hopkins University (JHU) must be identified as the RP.
Benefits of assigning JHU as the RP:
- Careful review of potential issues based on our prior experiences
- Reduction in the number of comments you will need to respond to
- We track the comments received to prevent future errors
- There is no added delay, only greater probability of success
You may also find the following CTgov-QCChecklist helpful
It is the policy of the organization that the following new or ongoing clinical trials shall be registered on http://clinicaltrials.gov:
1. Clinical Trials funded either in whole, or in part by National Institutes of Health (NIH) (applicable to all NIH-funded studies independent of whether the study meets the definition of an applicable clinical trial as detailed below):
The simplified case studies apply the following four questions to determine whether NIH would consider the research study to be a clinical trial:
- Does the study involve human participants?
- Are the participants prospectively assigned to an intervention?
- Is the study designed to evaluate the effect of the intervention on the participants?
- Is the effect being evaluated a health-related biomedical or behavioral outcome?
If the answer to all four questions is “yes,” then the clinical study would be considered a clinical trial according to the NIH definition.
Note that If the answers to the 4 questions are yes, your study meets the NIH definition of a clinical trial, even if…
- You are studying healthy participants
- Your study does not have a comparison group (e.g., placebo or control)
- Your study is only designed to assess the pharmacokinetics, safety, and/or maximum tolerated dose of an investigational drug
- Your study is utilizing a behavioral intervention
Note that studies that involve secondary research with biological specimens or health information are not clinical trials.
2. Trials that meet the clinical trial definition of The International Committee of Medical Journal Editors (ICMJE) that the investigator may wish to publish:
- ICMJE journals will consider [for publication] trials beginning on or after July 1, 2005 only if registration occurred before the first patient was enrolled (“prospective registration”). http://www.icmje.org/about-icmje/faqs/clinical-trials-registration/
3. Qualifying clinical trials which will render claims for items and services to the Center for Medicare and Medicaid Services (CMS):
- The National Clinical Trial (NCT) number must be included on claims for items and services provided in clinical trials that are qualified for coverage as specified in the “Medicare National Coverage Determination (NCD) Manual,” Section 310.1 https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/SE1344.pdf
4. Trials funded by major research funders and international non-governmental organizations (NGOs) in support of the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP)
On May 18, 2017 some of the world’s largest funders of medical research and international non-governmental organizations agreed on new standards that will require all clinical trials they fund or support to be registered and the results disclosed publicly.
They agreed to, “develop and implement policies within the next 12 months that require all trials they fund, co-fund, sponsor or support to be registered in a publicly-available registry.”
Dr Trevor Mundel, President, Global Health, Bill & Melinda Gates Foundation
“It’s a 21st-century best practice – and an essential part of the social contract that underlies medical research – that clinical trial data should be made publicly available less than one year after a clinical trial’s completion. We strongly support WHO’s effort to establish a global standard for reporting data within this timeframe, which is a practice we require of our grantees as well.”
5. Applicable Clinical Trials (ACT) which include the following:
- Trials of Drugs/Biologics: Controlled, clinical investigations of a product subject to FDA regulations. This includes preliminary studies or phase I trials to be published in an ICMJE journal.
- Trials of Devices: Controlled trials with health outcomes, other than small feasibility studies, and pediatric post-market surveillance.
Applicable Clinical Trials generally include interventional studies (with one or more arms) of FDA-regulated drugs, biological products, or devices that meet one of the following conditions:
- The trial has one or more sites in the U.S.
- The trial is conducted under an FDA Investigational New Drug Application (IND) or Investigational Device Exemption (IDE) application
- The trial involves a drug, biologic, or device that is manufactured in the U.S. or its territories and is exported for research
The following trials are generally excluded (unless funded either in whole, or in part by NIH):
- (Non-serious/life-threatening) Phase 1 drug trials, including studies in which drugs are used as research tools to explore biological phenomena or disease processes
- Small clinical trials to determine the feasibility of a device or a clinical trial to test prototype devices where the primary outcome measure relates to feasibility and not to health outcomes
- Trials that do not include drugs, biologics, or devices (e.g., behavioral interventions)
- Non-interventional (observational) clinical research, such as cohort or case control studies
- Trials that were ongoing* as of September 27, 2007, and reached the Completion Date before December 26, 2007
(*An ‘ongoing’ trial has enrolled one or more subjects and the final subject has not been examined or received an intervention for the purpose of collecting data on the primary outcome).
Checklist for Evaluating Whether a Clinical Trial or Study is an Applicable Clinical Trial (ACT)
The Johns Hopkins Principal Investigator (PI) should consult with commercial sponsors to assure that posting of a trial is in accord with terms of the study contract. A Sponsor providing drug only generally does not accept the registration and results reporting responsibilities. Generally for IND or IDE studies, the responsibility rests with the local investigator.
When to Submit Results?
- No later than 12 months after (Primary) Completion Date.
- Primary Completion Date FDAAA (Required for records first released on or after December 1, 2012)
- Date that the final subject was examined or received an intervention for purposes of final data collection for the primary outcome, whether the trial concluded per protocol or was terminated.
- Must keep this field accurate in ClinicalTrials.gov since it is how NIH determines the timeliness of basic results reporting.
- Must be updated not later than 30 calendar days after a change occurs
- Study Completion Date
- Final date on which data was (or is expected to be) collected.
- Source: https://prsinfo.clinicaltrials.gov/definitions.html#PrimaryCompletionDate
Tips for Entering Results:
- Ensure that the Enrollment # in the protocol section does not conflict with the # of participants Started in the Participant Flow module.
- Provide brief but informative Arm/Group titles.
- Ensure that the Arm/Group Description is not used to provide additional details about the interventions administered *(e.g., dosage, dosage form, frequency of administration) or groups evaluated.
- Ensure each Outcome Measure Title is a tool or method used to make a measurement, and not what will be measured how (e.g. “Pain Scores as measured by the Visual Analog Scale”).
- Ensure the number of participants at risk for “All-Cause Mortality” and “Serious Adverse Events” is equal to the number in the “Participant Flow” module.
- Ensure that each Outcome Measure should be specific and measurable by the units of measure provided.
- Provide a specific time-frame for each Outcome measure. (e.g., “End of study, Up to 3 months”). A common exception to this is a measure assessing change between two time points (e.g., “Change from Baseline Systolic Blood Pressure at 6 months”).
- Use the “Spelling” feature to ensure correct spelling and to expand all acronyms and abbreviations the first time used (and include acronym in parentheses). e.g. “National Institutes of Health (NIH)”.
- If the record is sent back with comments ensure all reviewer comments are addressed.
You may also find the following CTgov-QCChecklist helpful
Legacy Studies
When entering Basic Results for legacy studies (i.e. studies starting 2007 and earlier) with limited data accessibility or older studies that changed significantly from the time that they were first registered, one may attempt to punt via adding a link to the publication preceded by the following descriptive text (edit text as needed) under the “Basic Results/Limitations and Caveats” section (note: there is no guarantee this will be accepted by the ClinicalTrials.gov office but it’s worked in the past for some SKCCC trials):
“The raw study data is no longer available for this study [or] the design of this trial changed significantly from when it was first registered. The study’s publication can be accessed here: [www.webaddresshere.com].”
Terminated Studies
How do I submit results information if the trial is terminated (that is, stopped prematurely) and no data were collected for one or more Outcome Measures?
- If no participants were ever enrolled in the trial, set the Overall Recruitment Status to Withdrawn, and no further results information will need to be submitted.
- For a trial that was terminated after participants were enrolled, provide any available data. If no data are available for any of the Outcome Measures, specify zero (“0”) for the Number of Participants Analyzed in each Arm/Group, and leave the data fields blank. Provide an explanation in the Analysis Population Description or the Limitations and Caveats module.
NIH is launching a series of initiatives that are rolling out in 2017-2018 to enhance the accountability and transparency of clinical research. These initiatives target key points along the whole clinical trial lifecycle from concept to results reporting. Learn more about these changes and how they will affect your research.
Key Dates
- September 25, 2017 – New FORMS-E Application Instructions available on the How to Apply – Application Guide website
- October 25, 2017 – FORMS-E Application Packages will start being published for FOAs with due dates on or after January 25, 2018.
- Note that all new application packages will be published at least 60 days ahead of the first due dates
- January 25, 2018 – First due dates for new FORMS-E Application Packages. Responses to Requests for proposals issued as of this date must use the PHS Human Subject and Clinical Trial Information form for all proposals involving human subjects.
- Why Changes to Clinical Trial Policies?
- Good Clinical Practice Training
- Clinical Trial-specific Funding Opportunities
- Clinical Trial-Specific Review Criteria
- New Human Subject and Clinical Trial Information Form
- Single IRB Policy for Multi-site Research
- Clinical Trials Protocol Template
- Clinicaltrials.gov Registration and Reporting
- Clinical Trials – Frequently Asked Questions
- NIH Policy on the Dissemination of NIH-Funded Clinical Trial Information
- G.500 – PHS Human Subjects and Clinical Trials Information (Released September 25, 2017)
- 2.5 FTEs of dedicated staffing
- Statistical expertise
- Provided by the BEAD Core http://jhcchr.org/bead
- First 1-hour covered by the program
- Most concerns can be handled in the 1-hour consultation
- Additional time needed and costs can be discussed
- Study team assistance
- Registration
- Account creation and maintenance
- Initial registration
- Required for Applicable Clinical Trials
- Required for any clinical trial receiving full or partial NIH funding
- PRS reviewer comments (now time-limited to 15 calendar days)
- Update reminders (verification required every 12 months regardless of changes)
- Results reporting
- Changes to PI/Study team (including when a PI leaves)
- Results Reporting
- Results reporting reminders sent 3-4 months in advance (due 12 months after primary completion date)
- Assistance with results reporting
- Assistance with PRS reviewer comments (now time-limited to 25 calendar days)
- Registration
Use the following four questions to determine the difference between a clinical study and a clinical trial:
- Does the study involve human participants?
- Are the participants prospectively assigned to an intervention?
- Is the study designed to evaluate the effect of the intervention on the participants?
- Is the effect being evaluated a health-related biomedical or behavioral outcome?
Note that If the answers to the 4 questions are yes, your study meets the NIH definition of a clinical trial, even if…
- You are studying healthy participants
- Your study does not have a comparison group (e.g., placebo or control)
- Your study is only designed to assess the pharmacokinetics, safety, and/or maximum tolerated dose of an investigational drug
- Your study is utilizing a behavioral intervention
Studies intended solely to refine measures are not considered clinical trials.
Studies that involve secondary research with biological specimens or health information are not clinical trials.
Resources to Clarify the Definition
Case Studies
These simplified case studies illustrate the differences between clinical trials and clinical studies.
FAQs
These FAQs further clarify the application of the clinical trial definition.
Decision Tree
Print this decision tree for an easy reference for the four questions that identify a clinical trial.
Related Guide Notice
NOT-OD-15-015 Notice of Revised NIH Definition of “Clinical Trial”
- Final 2018 budget bill eases biomedical researchers’ policy worries – sciencemag.com, 3/22/2018
- Congress Stops NIH From Implementing New Clinical Trials Policy – Association for Psychological Science, 3/22/2018
- NIH Clinical Trials Report Language: What’s Next? – FABBS, 3/30/2018
NIH Final Rule September 16, 2016
- Federal Register Notice: HHS Final Rule
- Federal Register Vol. 81, No 183, September 21, 2016
- Federal Register Notice: NIH Policy
- Summary Table: HHS Final Rule and NIH Policy
- Summary of Changes: HHS Final Rule and NIH Policy
- JAMA: Toward a New Era of Trust and Transparency in Clinical Trials
- NEJM: The Final Rule for US Clinical Trial Registration and Results Information Submission
- NIH Director’s Blog: Clinical Trials – Sharing of Data and Living Up to Our End of the Bargain
- NIH Policy on the Dissemination of NIH-Funded Clinical Trial Information
Additional Information
- How to Submit Your Results homepage http://gov/ct2/manage-recs/how-report
- Basic Results Data Elements Definitions http://clinicaltrials.gov/results_definitions.html
- 10 minute webinars for each results module http://gov/ct2/manage-recs/present
- Helpful Hints (with common study designs examples) http://clinicaltrials.gov/ResultsExamples.pdf
- ACT Wizard: http://grants.nih.gov/clinicaltrials_fdaaa/docs/Flow_chart-ACT_only.pdf
- ClinicalTrials.gov history: https://www.clinicaltrials.gov/ct2/about-site/history
- ClinicalTrials.gov homepage: https://www.clinicaltrials.gov/
- ClinicalTrials.gov FAQ: https://clinicaltrials.gov/ct2/manage-recs/faq
- NIH Guidance on FDAAA: http://nih.gov/grants/guide/notice-files/NOT-OD-08-014.html
- FDA Guidance on Form FDA 3674: http://fda.gov/RegulatoryInformation/Guidances/ucm125335.htm
- Elaboration of Definitions of Responsible Party and Applicable Clinical Trial: https://clinicaltrials.gov/ElaborationsOnDefinitions.pdf
- HHS takes steps to provide more information about clinical trials to the public https://www.nih.gov/news-events/news-releases/hhs-takes-steps-provide-more-information-about-clinical-trials-public
Other Publications
- Anderson ML, Chiswell K, Peterson ED, Tasneem A, Topping J, Califf RM. Compliance with Results Reporting at ClinicalTrials.gov. N ENGL J MED 2015; 372:1031-1039 MARCH 12, 2015 DOI: 10.1056/NEJMSA1409364. Available at. http://nejm.org/doi/full/10.1056/NEJMsa1409364
- Chen R, Desai NR, Ross JS, Zhang W, Chau KH, Wayda B, Murugiah K, Lu DY, Mittal A, Krumholz HM. Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers. BMJ 2016 January 19;352:i637 [PDF]
- Gopal AD, Desai NR, Tse T, Ross JS. Reporting of noninferiority trials in ClinicalTrials.gov and corresponding publications. JAMA. 2015 Mar 17;313(11):1163-
- Mayo-Wilson E, Heyward J, Keyes A, Reynolds J, White S, Atri N, Alexander C, Omar A, Ford DE, on behalf of the National Clinical Trials Registration and Results Reporting Taskforce Survey Subcommittee. Clinical trial registration and reporting: A survey of academic organizations in the United States. BMC Medicine. 2018;16:60. https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-018-1042-6.
- O’Reilly EK, Hassell NJ, Snyder DC, et al. ClinicalTrials.gov Reporting: Strategies for Success at an Academic Health Center. Clinical and Translational Science. 2015;8(1):48-51. doi:10.1111/cts.12235. Available at. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4329023/
- Pillar C. Failure to Report: A STAT Investigation. STAT. 2015. Available at http://www.statnews.com/2015/12/13/clinical-trials-investigation/
- Williams RJ, Tse T, DiPiazza K, Zarin DA. Terminated trials in the ClinicalTrials.gov results database: Evaluation of availability of primary outcome data and reasons for termination. PLoS One. 2015 May 26;10(5):e0127242. [Full Text]
- Zarin DA, Keselman A. Registering a clinical trial in ClinicalTrials.gov. Chest. 2007;131(3):909-12
- Zarin DA, Tse T. Sharing individual participant data (IPD) within the context of the trial reporting system (TRS). PLoS Med. 2016 Jan 19;13(1):e1001946. [Full Text]
- Zarin DA, Tse T, Ross JS. Trial-results reporting and academic medical centers. N Engl J Med. 2015 Jun 11;372(24):2371-2. [Full Text]