Research Navigators help direct investigators to the ICTR services they need and advises them on “next steps” in the clinical and translational research enterprise at Johns Hopkins.
Research Navigator Services
Select examples of frequently requested assistance include:
- Referral to appropriate ICTR (and JHU) resources, services, and programs
- Referral to work with our partners at the University of Maryland ICTR (UMB ICTR)
- Direction to applicable Institutional policy and guidance
- Direction to help and navigation resources for users of ICTR and institutional electronic systems (e.g. Qualtrics, OnCore, CRMS, eIRB, CRUOnline)
- Direction to Human Subjects Training information and training requirements
- Providing general information about internal and external funding opportunities
- Providing educational sessions for divisions, departments and individual study teams about the resources available within the ICTR and across JHU to support specific research programs and projects
The Research Navigator’s Corner
February 2024
The Navigators regularly receive questions covering a wide range of topics about conducting research studies at Johns Hopkins. It is not uncommon for multiple investigators to submit the same query or raise issues that others in the research community would want to be aware of. Among the 15 inquiries that were submitted to the Navigators during the month of February, we believe that the following would be of interest to you:
My study will be enrolling healthy volunteers, who will be required to provide blood samples. How can I arrange to have these blood draws done?
With a population of healthy participants, there are 3 options for obtaining research bloods:
Phlebotomy services for both pediatric and adult patients are offered at designated CRU locations.
Research coordinators who can be hired on a part-time basis through the RCSS, receive some training in clinical skills, which includes phlebotomy.
Your own study team members can receive training in phlebotomy skills through this program.
Please navigate to each program’s website for information about:
- Contacts for questions
- Current fees and charges for using the provided services
- Submitting a request to obtain services
The Navigators regularly receive questions covering a wide range of topics about conducting research studies at Johns Hopkins. It is not uncommon for multiple investigators to submit the same query or raise issues that others in the research community would want to be aware of. Among the inquiries submitted to the Navigators during the month of January, we believe that the following would be of interest to you:
I am using eFormS to submit my secondary research protocol for JHM IRB approval. Even with the sample text and examples provided in the “instructional template” version of this form, I’m still not certain if I am using that information to correctly describe what will be happening in my study. Is there someone who can help me?
Because the IRB is responsible for determining which regulatory requirements will apply to the data being used in your study, their staff are in the best position to provide you with the most accurate responses to your questions.
You can submit a request to arrange a complimentary, one-on-one meeting with one of the IRB staff to discuss this matter here.
The Navigators regularly receive questions covering a wide range of topics about conducting research studies at Johns Hopkins. It is not uncommon for multiple investigators to submit the same query or raise issues that others in the research community would want to be aware of. Among the inquiries that were submitted to the Navigators during the month of December, we believe that the following would be of interest to you:
I am planning on using services offered by the ICTR on my next grant. How can I request a letter of support to include in the application?
- From any ICTR webpage, navigate to the left side of the lower border on that page and select the “Letters of Support” button.
NOTE: This link may not work if using a computer that cannot operate behind the Hopkins firewall.
- Complete the Request for ICTR Letter of Support for Grant Applicants form that uploads and select the “Submit” button when finished.
- Within five business days you will be emailed a link to your printable letter.
- From any ICTR webpage, navigate to the left side of the lower border on that page and select the “Letters of Support” button.
It is not uncommon for multiple investigators to submit the same query or raise similar issues that others in the research community would want to be aware of. Here are our top inquiries for the month of November that we believe would be of interest to you:
1. “How do I get help from a biostatistician?”
- Consultations with the Biostatistics and Study Design Program are requested by completing the form provided in the ICTR Service Request Portal.
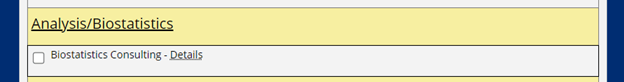
- As shown below, on the second half of the form, prior to the submit button, select “Biostatistics Consulting” under the “Analysis/Biostatistics” heading.
2. “I submitted a request for a consultation with the Biostatistics and Study Design Program, but no one has gotten back to me yet.”
- The ICTR Navigators can confirm if your request was successfully placed through the ICTR Service Request Portal.
- Waiting at least 7-10 business days for a response can occasionally occur when a larger than expected number of consultation requests are placed.
- A Research Navigator will follow-up with the program’s administrative unit to inquire about any delays lasting longer than 10 business days.


