Johns Hopkins Medicine is committed to making the experience of being a research participant as positive as possible.
Every six months since 2016, we identify a random sample of 500 research participants to receive a survey asking about their experience participating in a research study. In the survey, we ask about the informed consent process, interaction with the research team, and how easy it was to complete the study. We pledge to all who completed the survey that the results will be posted for the public and patients to review. The survey results are available through the links below.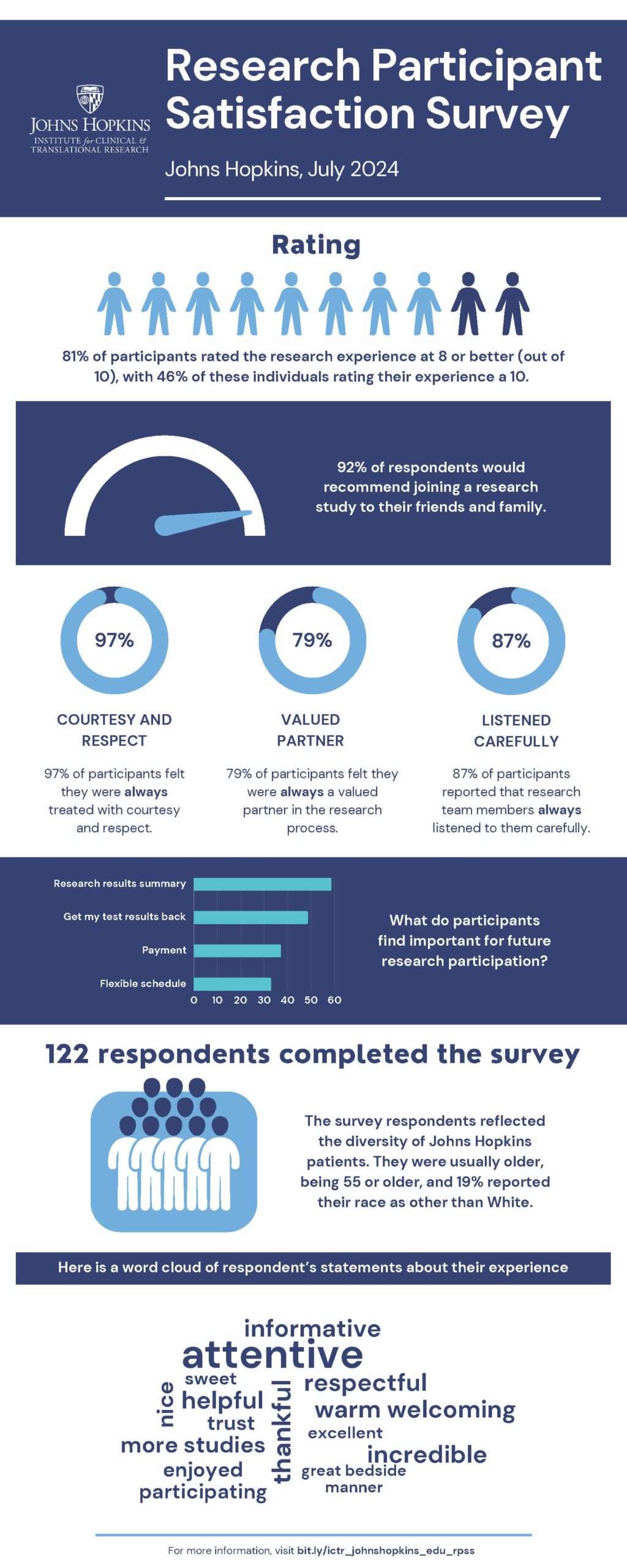
Download the July 2024 Results
Survey Distribution Methods
Every 6 months the CCDA (Center for Clinical Data Analysis) queries the CRMS (Clinical Research Management System) system for adult research participants who have been enrolled in a research project at any Johns Hopkins site or JH affiliate site in the previous 2-6 months to receive a satisfaction survey, described below.
CCDA cross-references this list with the Epic system to determine participants that have an email address in Epic. From the list of participants with email addresses on file, 500 are randomly selected to receive the survey. After random selection, the list is shared on a JH safe file server with a single study team member, who is responsible for sending out the survey. The email is sent via REDCap. The survey responses are also collected and stored on REDCap.
A repeat survey invitation is sent out to non- responders after two weeks of the original mailing.
Prior Survey Results
| January 2024 Results | July 2023 Results |
| January 2023 Results | July 2022 Results |
| January 2022 Results | August 2021 Results |
*September 2017(Conducted by Research America to determine the factors that contribute to a lack of minority participation in clinical trials.)
This survey was adapted from Kost et al Clin Trans Sci 2014 Dec;7(6):430-40. Any publication including mention of the use of the Instruments shall include appropriate reference to their origin or citation when available, and acknowledgement of grant number UL1 TR000043 as follows: “Supported in part by grant # UL1 TR000043 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program.”
The Empowering the Participant Voice project is supported in part by a Collaborative Innovation Award from the National Center for Accelerating Translational Science #U01TR003206.


