Clinical trials are tough and we want to support you to ensure that your trial is successful. Submit a TAP intake form to get connected with us. There is no charge for the initial consult.
What Can Working With the TAP Do for You?
We will support you in trial design, strategic operational planning, and leveraging all the resources of the ICTR. We work with trials funded by the NIH, commercial sponsors, or foundations.
The TAP is committed to facilitating the development of investigative expertise. K-grant, T-grant, and other early career investigators are invited to participate in project evaluation, trial simulation, and planning activities. Biostatistical doctoral and postdoctoral candidates may participate in statistical analysis plan development and reporting.
The TAP provides expert consultative support to investigators with small, local multi-center translational studies needing to:
- Improve research study design, trial operations, and analysis plans.
- Explore opportunities for single and multi-center trial innovation.
- Improve diversity and community engagement in clinical trials.
- Assess translational pathway and readiness for multi-center trials.
- Provide strategic assistance with grant applications.
- Develop and improve the overall stewardship, efficiency, accountability, and transparency of clinical trials.
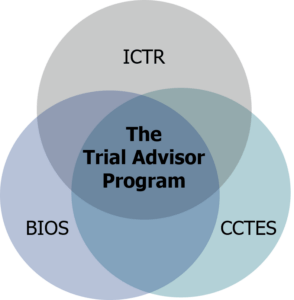
The TAP is composed of two key organizational partners—the ICTR and the BIOS Clinical Trial Coordinating Center (BIOS CTCC), a JHU-based academic research organization. A collaborative initiative, the TAP leverages the expertise, skills, and knowledge of ICTR and BIOS CTCC faculty to ensure the best possible outcomes for the trials we support. The TAP supports innovation in clinical trials and studies while providing meaningful scientific review to ensure rigorous, informative research.
The TAP Process Flow
The easy 4-step process shown below supports investigators in their trial needs.
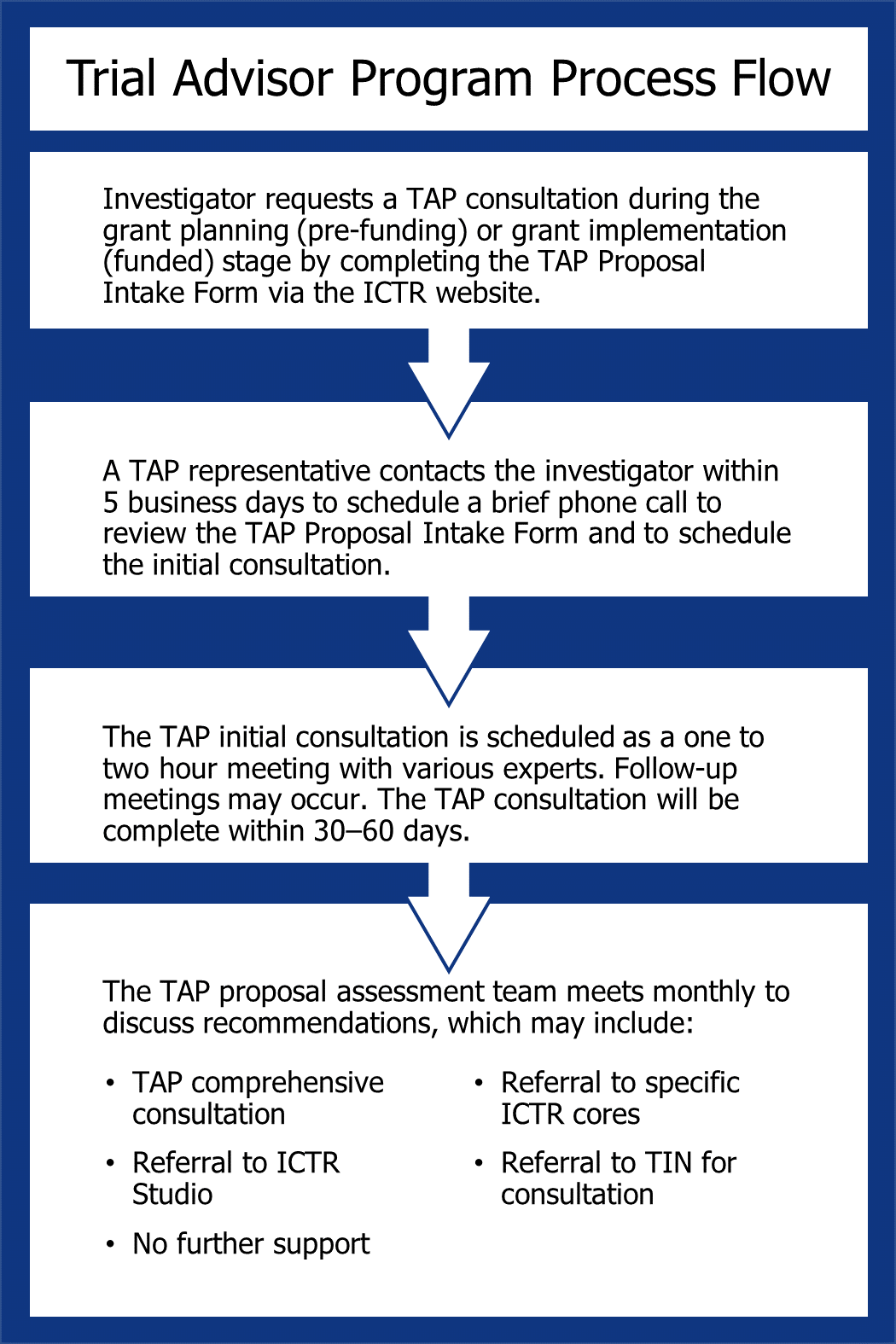
The TAP consultative process provides study teams with referrals to experts in their fields and may connect investigators to services which may include any or all of the following components:
- Cohort discovery via PCORNet and TriNetX mechanisms
- Site selection and readiness assessment within and beyond the Johns Hopkins Clinical Research Network
- Single IRB (sIRB) review in collaboration with the regulatory knowledge and support core
- Review method development to track study performance and outcomes at the local hub and affiliated sites
- Organization and trial execution strategy consultation
- Direction and strategy for grant preparation
TAP Associated Costs
The TAP consultative process is free to you and funded by the current CTSA grant from the National Center for Advancing Translational Sciences (NCATS).
The TAP representative will organize an initial meeting of 1 hour for the investigator with experts from the ICTR and the BIOS CTCC.
After the initial meeting, the TAP service provides an additional hour of free support for the research project to provide supporting material and/or further planning discussed during the initial meeting. The initial TAP 2 hour consult is free. If you need specific grant services, planning or additional support after the 2 hour consult, a broad spectrum of services are available through the TAP teams for a fee.
TAP Leadership and Administrative Team Members
Here are some of the people that can help you navigate the entire grant process. Our leadership are experts in:
- Multi-center clinical trials
- Trial start-up, enrollment, data quality, GCP, IRB and sIRB processes, contracting, human subjects research
- Leadership and management of research teams
- Use of performance metrics
- Comprehensive safety & risk management
- Local research conduct

DANIEL FORD, MD, MPH
Director, PI, ICTR
Senior Associate Dean for Clinical and Translational Research, School of Medicine

DAN HANLEY, MD
Deputy Director, ICTR
Division Director, BIOS Clinical Trial Coordinating Center, School of Medicine

DOUGLAS A. JABS, MD, MBA
Director, Center for Clinical Trials and Evidence Synthesis

KAREN BANDEEN ROCHE, PHD
Director of Biostatistics, Epidemiology and Research Design (BERD), ICTR
Program Director, Biostatistics Center

CHARLES FLEXNER, MD
Chief Scientific Officer for Strategy and Integration

NICHOL MCBEE, MPH
Research Associate, Department of Neurology
Division Manager, BIOS CTCC
Administrator, TAP

LIZ MARTINEZ, RN, BSN, CCRC
Research Participant Advocate
Senior Clinical/Research Liaison, ICTR

MEGHAN HILDRETH, MS
Senior Research Program Coordinator, BIOS CTCC
Senior Research Program Coordinator, TAP

ANGELINE NANNI, MBA, MS
Research Navigator, BIOS CTCC
Research Navigator, TAP

TAYLOR M. BINNIX, MA, MPH
Research Program Manager, Center for Clinical Trials and Evidence Synthesis
Make a Request
All requests must be submitted through the ICTR Service Portal.
For questions regarding a current project, please email [email protected].


