Research Navigators help direct investigators to the ICTR services they need and advises them on “next steps” in the clinical and translational research enterprise at Johns Hopkins.
Research Navigator Services
Select examples of frequently requested assistance include:
- Referral to appropriate ICTR (and JHU) resources, services, and programs
- Referral to work with our partners at the University of Maryland ICTR (UMB ICTR)
- Direction to applicable Institutional policy and guidance
- Direction to help and navigation resources for users of ICTR and institutional electronic systems (e.g. Qualtrics, OnCore, CRMS, eIRB, CRUOnline)
- Direction to Human Subjects Training information and training requirements
- Providing general information about internal and external funding opportunities
- Providing educational sessions for divisions, departments and individual study teams about the resources available within the ICTR and across JHU to support specific research programs and projects
The Research Navigator’s Corner
The Navigators regularly receive questions covering a wide range of topics about conducting research studies at Johns Hopkins. It is not uncommon for multiple investigators to submit the same query or raise issues that others in the research community would want to be aware of. Among the inquiries submitted to the Navigators, we believe that the following would be of interest to you.
JHM Language Services provides communication assistance for all patients (including those participating in research studies) who do not speak English; have Limited English Proficiency; are Deaf or Hard of Hearing; have Low Vision or a Speech Disability.
Requests for assistance can be submitted as follows:
Language interpretation requests can be submitted by:
- Phoning 410-614-4685 (TTY: 711) (24/7, 365 days a year)
- *Completing the webform available at https://jhmiportal.force.com/LASWebRequestFormInterpretation
- *Emailing [email protected]
*Should not be used when services are required within 24 hours
Document translation requests can be submitted by:
- Phoning 410-614-4685 (TTY: 711) (24/7, 365 days a year)
- Completing the web form at https://jhmicrm.my.site.com/LASWebRequestFormTranslation
- Emailing: [email protected]
More information about these services can be obtained by :
- Phoning 410-614-4685 (TTY: 711) (24/7, 365 days a year)
- Emailing [email protected]
- Consulting the JHM Language Services website at https://intranet.insidehopkinsmedicine.org/jhm-language-services/interpretation-translation.html
Clinical Research Support Services (CRSS), an office within the Johns Hopkins SOM Office of Research Administration, can provide this help. A detailed description of their Clinical Trial Review Process can be found here.
There are 2 major steps:
- Determine if a Prospective Reimbursement Analysis (PRA) is required for a study and carry out the analysis when needed. The purpose of the PRA is to identify study related cost and whether or not they are billed to the sponsor or the patient
Every protocol reviewed by the Johns Hopkins Medicine IRB is subject to a PRA determination, though teams may email [email protected] to request that this step be started prior to eIRB submission.
Study-related documentation that must be available for CRSS review during the PRA process includes but is not limited to: the protocol; investigator’s drug brochure; informed consent form; and contract or grant.
2. Draft the clinical budget, including PRA costs when applicable, and negotiate final approval with the sponsor
The team first reviews the CRSS provided Budget Checklist and CRSS Budget Template to identify the information that must be available to develop a budget.
After all the information that is required to create a study budget per the CRSS’ Budget Checklist and Budget Template has been obtained, a request for assistance with budget development and negotiation is emailed to [email protected].
CRSS’ draft budget will be forwarded to the Principal Investigator and study team for review and approval. Upon internal budget approval, negotiations will begin with the PI and study team copied on all correspondence to the sponsor.
Questions and/or concerns related to Prospective Reimbursement Analyses and/or budget development should be directed to Clinical Research Support Services at 410-361-8372 or [email protected].
Two options are available to identify potential candidates for this type of position:
- The Research Coordinator Support Service (RCSS).
Formerly known as the Study Coordinator Apprenticeship and Mentoring Program (SCAMP), RCSS is a fee for service program (see RCSS site for current rates) that provides research coordinator support on a temporary basis. RCSS coordinators are trained to manage a wide range of responsibilities which can be customized to best fit the needs of individual research teams.
Requests for support are submitted via the ICTR Service Request Portal.
Questions about using this service can also be submitted to the ICTR Service Request Portal, as well as emailed to Anthony Keyes, MBA, Assistant Director ([email protected]) or Mais Hamdawi, MD, Sr. Research Program Manager, ([email protected] )
2. Medasource
Medasource is a Clinical Research consulting and support firm that includes Johns Hopkins among the over 150 research institutions, health systems, academic medical centers, and pharmaceutical companies with whom they partner to provide short-term and long-term support in a wide range of areas. The expertise that is available includes but is not limited to :
- Research administration (pre and post award)
- Clinical Trial Project Management
- Clinical Trial Contracts and Finance
- Regulatory compliance
- Medical and Scientific writing
- Data management
The workflow followed to hire staff from Medasource, is shown here.
All questions about using this resource should be directed to the Office of Clinical Trials at [email protected]
Information about generating reports with SlicerDicer can be obtained from 2 sources:
- The “Fundamentals of SlicerDicer” E-Learning course is accessed from the myLearning website.
This course takes approximately 30 minutes and is designed to familiarize the user with basic SlicerDicer features and functionality, including:
- Populations
- Data Models
- Criteria
- Slices
- Measures
- Visual Options
- Creating a query
- Health IT maintains a comprehensive portfolio of resources (documents, PowerPoints and videos) that covers a wide range of topics associated with SlicerDicer reporting. It can be accessed from Health IT’s Education Portal on SharePoint. Select “Search the Portal” option and search using the keyword “Slicer”.
With a population of healthy participants, there are 3 options for obtaining research bloods:
Phlebotomy services for both pediatric and adult patients are offered at designated CRU locations.
Research coordinators who can be hired on a part-time basis through the RCSS, receive some training in clinical skills, which includes phlebotomy.
Your own study team members can receive training in phlebotomy skills through this program.
Please navigate to each program’s website for information about:
- Contacts for questions
- Current fees and charges for using the provided services
- Submitting a request to obtain services
Because the IRB is responsible for determining which regulatory requirements will apply to the data being used in your study, their staff are in the best position to provide you with the most accurate responses to your questions.
You can submit a request to arrange a complimentary, one-on-one meeting with one of the IRB staff to discuss this matter here.
- From any ICTR webpage, navigate to the left side of the lower border on that page and select the “Letters of Support” button.
NOTE: This link may not work if using a computer that cannot operate behind the Hopkins firewall.
- Complete the Request for ICTR Letter of Support for Grant Applicants form that uploads and select the “Submit” button when finished.
- Within five business days you will be emailed a link to your printable letter.
- From any ICTR webpage, navigate to the left side of the lower border on that page and select the “Letters of Support” button.
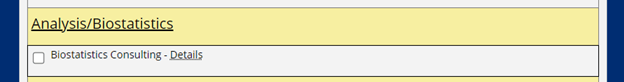
Consultations with the Biostatistics and Study Design Program are requested by completing the form provided in the ICTR Service Request Portal.
As shown below, on the second half of the form, prior to the submit button, select “Biostatistics Consulting” under the “Analysis/Biostatistics” heading.
- The ICTR Navigators can confirm if your request was successfully placed through the ICTR Service Request Portal.
- Waiting at least 7-10 business days for a response can occasionally occur when a larger than expected number of consultation requests are placed.
- A Research Navigator will follow-up with the program’s administrative unit to inquire about any delays lasting longer than 10 business days.
