
The MyChart Recruitment Service offers a secure method of electronically recruiting potential research participants.
Introduction
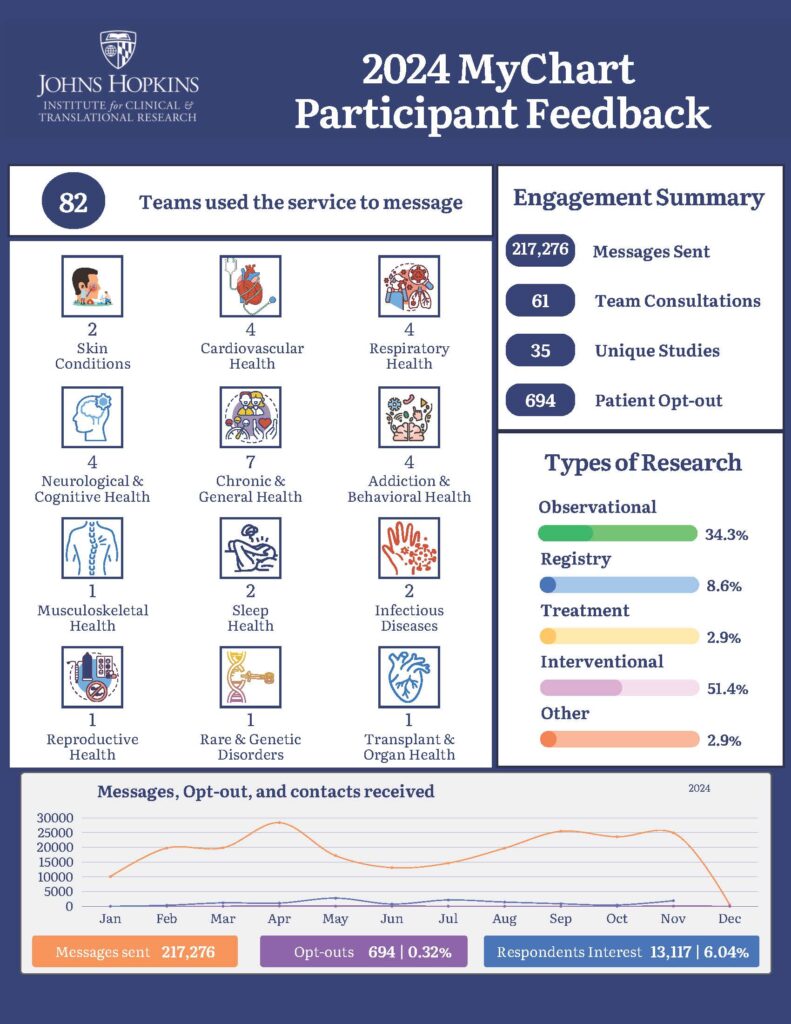
The MyChart Recruitment Service is a collaboration between data analysts, experts in recruitment methods, and clinical researchers that offers a secure method of electronically recruiting potential research participants. Target populations are identified and reached through database queries that are embedded in Epic. Potential participants are messaged via their activated Johns Hopkins MyChart account. Team members at the ICTR Recruitment Innovation Unit (RIU) work together to manage this service. Our goal is to support ongoing research at Johns Hopkins, while also empowering and engaging patients by connecting them with clinical research studies that may be of interest to them.
Study Team Satisfaction
Among the study teams that have utilized our service, 100% agreed that they would recommend the MyChart Recruitment Service to others or use it in future projects. Additionally, 100% of the teams reported that they were very satisfied with the quality of support provided by the Service.
How to Get Started
Inquiries to utilize the MyChart recruitment service should be sent to our Data Manager, Tosin Tomiwa, at [email protected]. Additionally, you have the option to submit a service intake form through this link: https://mrprcbcw.hosts.jhmi.edu/redcap/surveys/?s=4HDWKKLJNWTXRELE.
Please note that approval from our service committee is a prerequisite before proceeding with the submission to the IRB.
Our Publications
For more information on our Service/this recruitment method, please see our publications:
Gleason, K. T., Ford, D. E., Gumas, D., Woods, B., Appel, L., Murray, P., … Dennison Himmelfarb, C. R. (2018). Development and preliminary evaluation of a patient portal messaging for research recruitment service. Journal of Clinical and Translational Science, 2(1), 53–56. https://doi.org/10.1017/cts.2018.10
Miller, H. N., Gleason, K. T., Juraschek, S. P., Plante, T. B., Lewis-Land, C., Woods, B., … Dennison Himmelfarb, C. R. (2019). Electronic medical record-based cohort selection and direct-to-patient, targeted recruitment: Early efficacy and lessons learned. Journal of the American Medical Informatics Association, 26(11), 1209–1217. https://doi.org/10.1093/jamia/ocz168
Plante, T. B., Gleason, K. T., Miller, H. N., Charleston, J., McArthur, K., Himmelfarb, C. D., … Juraschek, S. P. (2020). Recruitment of trial participants through electronic medical record patient portal messaging: A pilot study. Clinical Trials (London, England), 17(1), 30–38. https://doi.org/10.1177/1740774519873657
Frequently Asked Questions
Follow these steps to recruit participants using MyChart:
Overview of the Project with the MyChart Recruitment Team:
- Submit a Request
- You will be contacted to complete a consultation with our team.
Intake with the Core for Clinical Research Data Acquisition (CCDA)
- Meet with the CCDA Team to identify what type of patients will be recruited and if this will be feasible.
Overview of Project by MyChart Council
- The Council meets once every month to review the requests received. They may approve or make recommended/required changes.
Approval from IRB
- When approved by the Council, submit a Change in Research (CIR) to the IRB, indicating the use of the MyChart Recruitment Service for recruitment. Make sure to include the MyChart approval letter, which you will get after the MyChart Council meets and approves your team and messaging letter.
CCDA Writes Query
- When the study is approved, send it to the MyChart recruitment team via email. The MyChart team will notify the CCDA team to proceed with query development.
Eligible Patient List is Deployed in EPIC
- After the query is developed, it is sent to the EPIC/PACE team for deployment in the EPIC system. This process usually takes about two weeks.
- Once the query is deployed, it generates a report in EPIC that enables the messaging team to view a list of patients.
- The study team will be notified once the query has been successfully deployed.
Patient Messaging:
- Once the EPIC study patient report is ready, the MyChart team will notify the study team, and set up and test the messaging frequency.
- Messaging is limited to 1000 messages per week.
- Note that patients who have opted out of future research contact cannot be included in the messaging.
When the MyChart recruitment team sends a message, patients who may meet eligibility criteria receive a notification within their MyChart patient portal indicating a new message is available. Upon logging in, patients will find the research invitation letter detailing the study and the reasons for reaching out to them. If interested, patients can complete a survey to share their contact information. Once submitted, the research team is alerted to the patient’s interest. The research team can then reach out to the patient regarding the study.
Patients who have an active MyChart patient portal account (active proxy account if under 18) with approval for receiving recruitment messages, and preferences for receiving notifications via email.
Study-specific eligibility criteria can be searched in the medical record, including but not limited to demographic information, ICD9/10 codes, lab values, billing data, and healthcare utilization patterns.
The number of individuals you can contact varies widely and depends on your study’s specific eligibility criteria. Historically, report sizes have ranged from as few as 200 patients to as many as 49,999 patients. The exact number for your study will be determined based on the inclusion and exclusion criteria as defined and created with the CCDA.
The average response rate is 7.04%. Effectiveness varies based on the population of interest and trial commitments:
- Entirely online studies: 8.66% response rate.
- Studies seeking healthy volunteers: 2.6% response rate.
Our service includes four main components, two of which are provided at no cost:
Initial Consultation with the MyChart Recruitment Team: This initial meeting is provided at no cost. During this consultation, we discuss your study and recruitment needs.
Consultation with the CCDA Team: This consultation, also provided at no cost, involves discussing your study query with the Clinical Research Data Analyst (CCDA) team. They will assist in building the study computable phenotype/query.
CCDA/PACE Services for Development and Deployment:
- CCDA charges $130 per hour for writing and developing the query. On average, query development takes about 14.5 hours.
- The deployment of the developed query in EPIC is done by the Program to Accelerate Clinical Research (PACE) team using EPIC (led by Matt Courtemanche). The fee is a flat fee of $250 and this fee covers query initial build in EPIC, documentation, testing, IT initiated maintenance and report retirement.
- For query modification after deployment, the PACE team charges $150 for the deployment of modified query.
Message Management by the MyChart Recruitment Team:
Our MyChart messaging service offers transparent and predictable pricing, with two main options tailored to your study’s messaging needs. All costs are prorated based on your usage, ensuring flexibility:
- Standard Batch Messaging
- Cost per Batch: $13 per batch of messages sent through MyChart
- Billing Schedule: Invoiced quarterly, based on actual number of batches sent.
- Customized Messaging
- Cost per Hour: $26 per hour for customizing and sending message through MyChart.
- Billing Schedule: Invoiced quarterly, based on actual number of hours spent messaging.
The majority of the work by the study team is completed in the preliminary steps of the process. After the team has IRB approval and the report is generated, the team will establish a timeline and calendar with the MyChart team members. From that point, the MyChart team will handle the sending of messages on a regular basis. A customized REDCap survey will be provided to each study team to track the inquiries.
The study team must be attentive to the patients who respond and show interest in the study.
A standard messaging schedule will be coordinated with the MyChart recruitment team at the outset. The frequency of messaging can be customized based on the size of the report. Study teams have the option to select messaging frequencies ranging from weekly to monthly, with the flexibility to adjust as needed during the course of the project.
To learn more about obtaining EPIC access for your team, click here or visit https://confluence.jh.edu/pages/viewpage.action?spaceKey=HIT&title=%28Research%29+User+Access+to+Epic.



