The Core for Clinical Research Data Acquisition (CCDA) assists researchers with accessing clinical data for research purposes. The CCDA is staffed with experienced data analysts who will assist you with access to data while also helping you comply with Data Trust privacy and security regulations.
The Core for Clinical Research Data Acquisition (CCDA) is one of the 10 Data Trust analytic teams responsible for assisting researchers with accessing clinical data for research. Additionally, we are one of the clinical research informatics cores under the ICTR.
- Preliminary, anonymous data for feasibility, grant applications and statistical population sample-size estimates.
- IRB-approved case-finding for study enrollment (mailings, phone solicitation), chart review, and cohort/case-control studies.
- Natural Language Processing service–Text mining and information extraction methods to identify disease, medications, symptoms, and signs from clinical text, as well as artificial intelligence techniques for sentiment analysis, opinion mining, measuring cognitive ability, and exploring social determinants of health.
- Research data extracts.
- Data de-identification services to confirm to HIPAA Privacy standards.
- Honest Broker services.
- Assistance using Epic’s SlicerDicer and TriNetX self-service tools.
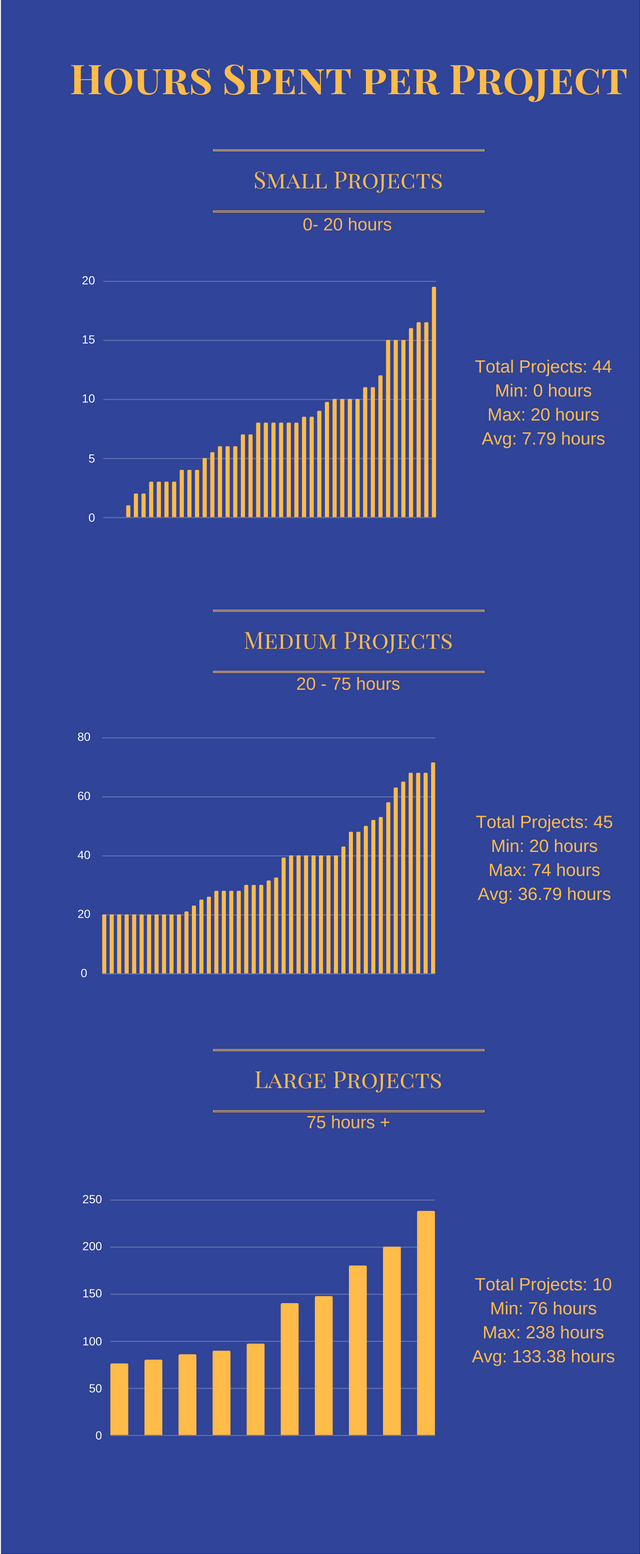
We have built an infrastructure that enables data through a secure, repeatable process that meets your needs, the institution’s values, and applicable privacy and security laws and policies. We looked at how comparable research institutions met this demand, and determined to pursue this approach. Similar to the 20+ other service centers at JHU such as the Genetic Core, the Biological Repository, and the Training and Management Development programs, we have developed a no-profit, revenue neutral approach that aims to provide high quality, lowest possible cost service to the research community. This hourly cost includes analysts, database administrators, servers, software, and management.
Analysis and Data Extraction Services for IRB Approved Studies:
- Effective July 1, 2024 $130/hour for new projects.
- The first 2 hours of guidance and feasibility review for new projects are free of charge
- Ongoing maintenance of established periodic extracts is factored into the cost
- Additional requests outside the scope of the initial project will be charged at the agreed upon hourly rate.
Analysis and Data Extraction Services for Quality Improvement Projects:
- Effective July 1, 2024 $130/hour for new projects.
- Rates apply for guidance and feasibility review of new projects
- Ongoing maintenance of established periodic extracts is factored into the cost
- Additional requests outside the scope of the initial project will be charged at the agreed upon hourly rate.
Analysis and Data Extraction Services for External Researchers and Entities (non-Johns Hopkins):
- Effective July 1, 2020 $182/hour
- Rates apply for guidance and feasibility review.
- Requests must be covered by an IRB-approved protocol, not for quality improvement or other purposes.
- Additional requests outside the scope of the initial project will be charged at the agreed upon hourly rate.
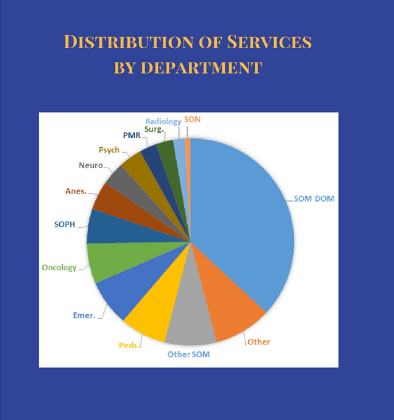
Our team is comprised of trained data analysts with access to multiple data sources including Epic, CaseMix/Data Mart, EPR2020 (Electronic Patient Record), Clinical Research Management System (CRMS), and Sunrise Clinical Manager (POE).
Frequently requested data elements include:
- Demographic data
- Encounters/Visits (dates/times, locations/clinics, visit providers)
- Diagnoses (problem list, recorded at the encounter, billed)
- Lab Results
- Medications (ordered, administered, patient-reported)
- Procedures (surgical, imaging, etc.)
- Flowsheet variables (vitals, etc.)
- Questionnaires (Depression screening, surveys)
To get started, you can submit a request to our team here: https://mrprcbcw.hosts.jhmi.edu/redcap/surveys/?s=A8FE8FT47W
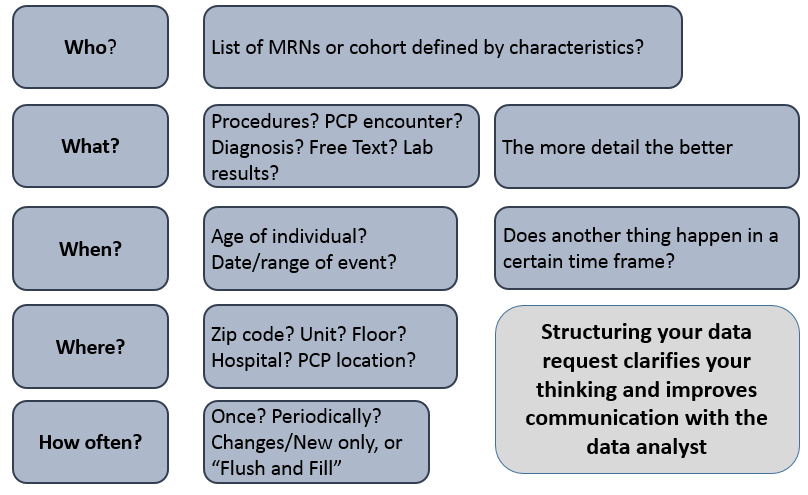
In order to prepare for your initial intake meeting, it is often helpful to consider the following components regarding the specific data elements of interest: who, what, when, where, and how often. Please see the graphic below for more information about how to turn your high concept into an actionable request!
As part of our Honest Broker responsibilities, the CCDA will only deliver data extracts to secure locations approved by the IRB and Data Trust. We recommend requesting a SAFE Virtual Desktop when specifying the location for PHI storage and analysis. The SAFE is a Windows 10 desktop installed with statistical software including R Studio, SAS, Stata, MS Office productivity suite (Excel, Word, Access), and Python. By using this virtual desktop, you can ensure that the data we provide will be secure.
For more information on data trust and the SAFE Desktop, please refer to the following other informational pages.