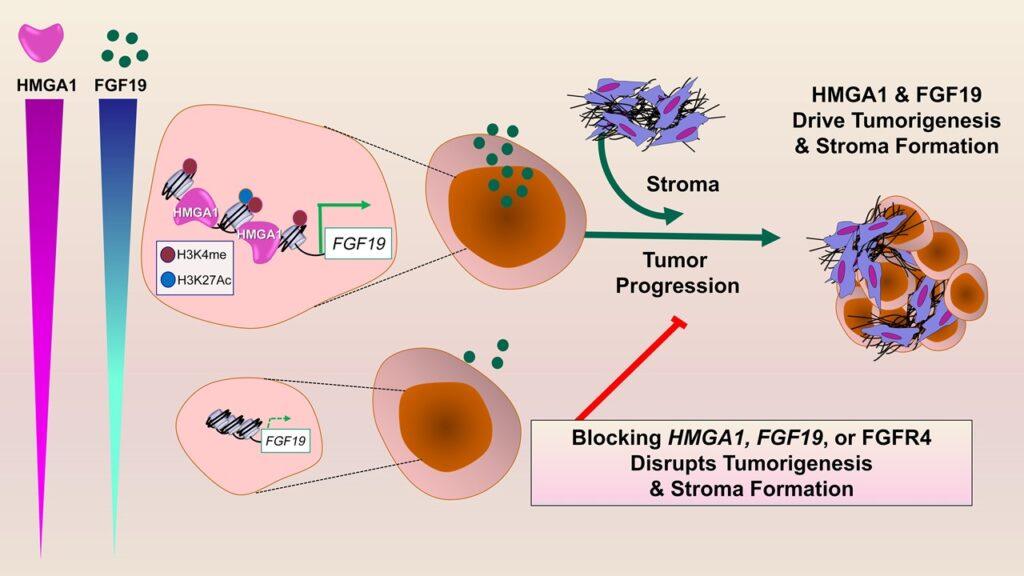
The High Mobility Group A1 (HMGA1) protein functions as a “molecular switch” that “flips on” expression and secretion of a critical growth factor, called FGF19, in pancreatic cancer. Together with HMGA1, FGF19 promotes tumor progression and formation of a dense wall-like structure called the “stroma”, which prevents therapy from reaching tumor cells. Furthermore, pancreatic tumors with high expression of HMGA1 and FGF19 comprise a subset of human pancreatic cancers with extremely poor outcomes. Most importantly, inhibitors to FGF19 receptors (FGFR4) are already available in the clinics for other diseases and provide a potential new therapy for these highly lethal pancreatic tumors. Credit: Bowen Wang and Linda Resar
A description of the work was published online March 15 in The Journal of Clinical Investigation.
“Pancreatic cancer is among the most recalcitrant tumors, for which there really are no effective therapies,” says senior study author Linda Resar, MD, professor of medicine, oncology and pathology at Johns Hopkins. Many patients succumb to pancreatic cancer in six to 12 months of diagnosis, she says, and genomic data indicates that patients with pancreatic cancers with high levels of both HMGA1 and FGF19 have the worse outcomes with even shorter survival than that of other patients with pancreatic cancer.
“In prior work, we found that HMGA1 was overexpressed in most pancreatic cancers and very late stage precursor lesions, as well as in other aggressive tumors such as leukemia and advanced stage myeloproliferative neoplasms, which suggested to us that HMGA1 was playing a fundamental role in driving tumor progression,” Resar says.
In a series of laboratory experiments, Resar and colleagues investigated several methods of disrupting HMGA1 and FGF19. First, they silenced HMGA1 in pancreatic cancer cell lines from primary and metastatic tumors using short hairpin RNA—or artificial RNA molecules that block gene expression—and observed that a deficiency in HMGA1 led to decreased growth rates, impairing migration, invasion and other cancerous properties. They also developed mouse models of pancreatic cancer missing one or two mouse genes for HMGA1 in the pancreas. Surprisingly, loss of just one gene was sufficient to slow tumor formation and progression.
In additional tests, researchers found that FGF19 gene expression, protein levels in pancreatic cancer cells and secretion all depend on HMGA1. Also, silencing FGF19 mirrored effects of silencing HMGA1, decreasing tumor growth and invasive properties. Most importantly, administration of BLU9931, a small-molecule drug that inhibits FGFR4 (a receptor for FGF19), led to decreased tumor growth and stroma formation in mouse tumors.
“Together, we discovered what we believe is a previously undescribed paradigm whereby tumor cells collaborate via HMGA1 and FGF19 to drive cancer progression and stroma formation,” Resar says. “This work also unveiled FGF19 as a potential therapeutic target for a very aggressive subset of human pancreatic cancers. Perhaps the most exciting aspect of our studies is that inhibitors to FGF19 are available and have already been tested in humans.”
In ongoing studies, the researchers are evaluating additional tumors to determine if they upregulate FGF19 and could benefit from treatment with FGF19 blockade. The researchers will also test whether inhibiting FGF19 blocks the spread or metastasis of tumor cells to distant sites.
Study co-authors are Lionel Chia, Bowen Wang, Jung-Hyun Kim, Li Z. Luo, Shuai Shuai, Iliana Herrera, Sophia Chen, Liping Li, Lingling Xian, Tait Huso, Mohammad Heydarian, Karen Reddy, Woo Jung Sung, Shun Ishiyama, Gongbo Guo, Elizabeth Jaffee, Lei Zheng, Leslie Cope, Kathy Gabrielson and Laura Wood.
The work was supported by the National Institutes of Health (grants R01 CA232741, R01 HL145780, R01 DK102943, R01 HL143818 and R03 CA182679), the Allegheny Health Network, the Maryland Stem Cell Research Fund and the Sol Goldman Pancreatic Cancer Research Center.