Article courtesy of The Baltimore Banner
This article discusses the work of Barbara Slusher, PhD, MAS, director of Johns Hopkins Drug Discovery, and co-leader of the ICTR Drug, Biologics, Vaccines and Devices Translational Research Community.
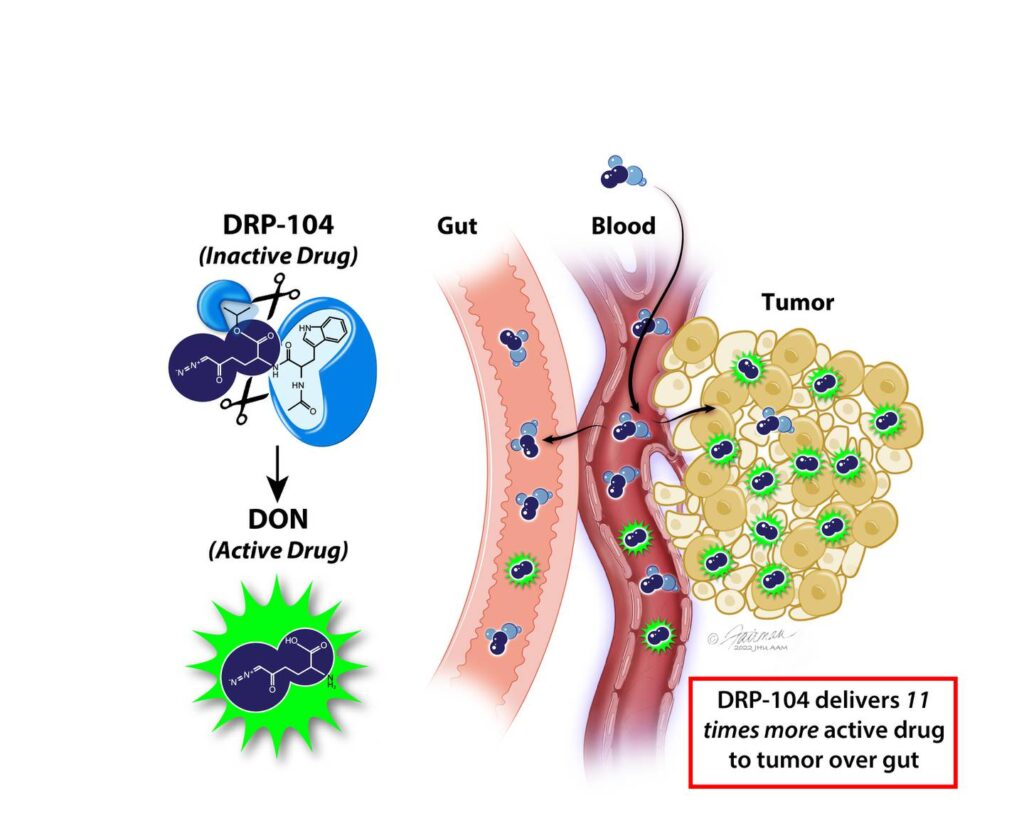
Chemotherapy has long been the go-to treatment for cancer, but the drugs aren’t always strong enough against advanced tumors. They also damage other parts of the body.
Some scientists at Johns Hopkins University say they may have discovered something better. They developed and have begun testing a new drug on cancer patients at the university’s Sidney Kimmel Comprehensive Cancer Center and elsewhere around the country.
The process started by chemically altering an extra-powerful drug that was abandoned decades ago when it was deemed too toxic for people. It killed cancer cells really well, but it also killed a lot of healthy cells, particularly in the gut.
“What’s novel here is we’ve made the drug inactive when it enters the body, only activating in the tumors,” said Barbara Slusher, PhD, MAS, director of the Johns Hopkins Drug Discovery, which researches and develops new drugs and repurposes existing ones.
“It’s active where we want it and inactive where we don’t want it,” she said. “We showed that when we give this prodrug we get 11 times more drug to the tumor than the gut.”
Prodrugs aren’t new. There are other such drugs in use in cancer treatments and other therapies, and they mostly improve how a drug is absorbed or targeted. But Slusher said the chemical alteration allows them to effectively turn the drug on and off in different parts of the body.
The drug is targeted to the tumors by its attraction to glutamine, a key nutrient fueling growth in certain cancer cells.
Other healthy cells also consume a lot of glutamine, such as those in the lining of the gut. That’s why the gastrointestinal tract was so hard hit by the drug before the Hopkins scientists altered it.
But the drug won’t do anything until the active ingredient is turned on. To do this, scientists attached chemicals called promoieties that make the drugs inert. The promoieties are clipped off by enzymes that are abundant in the tumors, but not in the gut.
Scientists at the company now testing the new drug, known as DRP-104, warn it’s still early in the process.
Tumors were all eliminated in mouse studies when the drug interfered with glutamine consumption, according to a report published Nov. 16 in the journal Science Advances. But the drug could fail in people. It’ll also take years to show it’s safe and works, although the U.S. Food and Drug Administration has given the drug a fast-tracked schedule.
The first trials are getting underway in people for whom traditional therapies have failed.
Dr. Mohamed Ragab, chief business officer at Dracen Pharmaceuticals — which was co-founded by Slusher and other Hopkins scientists — said if the trials show the drug works, it could be used alone or in combination with other treatments. Cancer patients typically have surgeries, radiation, chemotherapy.
Scientists believe the drug may be particularly effective in combination with immunotherapy, a more recently developed therapy that involves sparking a person’s own immune system to fight cancer. It’s considered a potent new tool but still doesn’t work in most cancer cases.
Ragab said Dracen’s research has uncovered genetic mutations that suggest which cancers the drug will work best against. The mutations make some tumors more dependent on glutamine and they are found in lung and advanced prostate cancers, among others.
Importantly for patients, Ragab said the drug could have other benefits besides sparing healthy body parts from damage. It could reduce some other unpleasant side effects common among cancer patients such as weight and hair loss.
“The phase 1 clinical trial to confirm safety is still ongoing and data are yet to be publicly disclosed, but suffice to say that at high therapeutic doses we see infrequent and non-severe side effects,” Ragab said.
The trials have gained attention in the scientific community, including from Dr. Timothy Yap, a clinical trials committee co-chair for the American Association for Cancer Research’s annual meeting in 2023.
“This is a novel and exciting strategy that exploits a unique Achilles heel of cancer, and has the potential to target tumors selectively, while not impacting normal cells,” said Yap, also medical director at the University of Texas MD Anderson Cancer Center’s Institute for Applied Cancer Science and an associate professor in the Department of Investigational Cancer Therapeutics.
“This has great potential to lead to an effective and well-tolerated drug for our patients,” Yap said.
