Helps clarify the benefit of chemotherapy in stage II cancers
A new research study showed that circulating tumor DNA (ctDNA) — genetic material shed from tumors into the bloodstream — can identify stage II colon cancer patients who can most benefit from chemotherapy following surgery and spare other patients the need for this form of treatment.
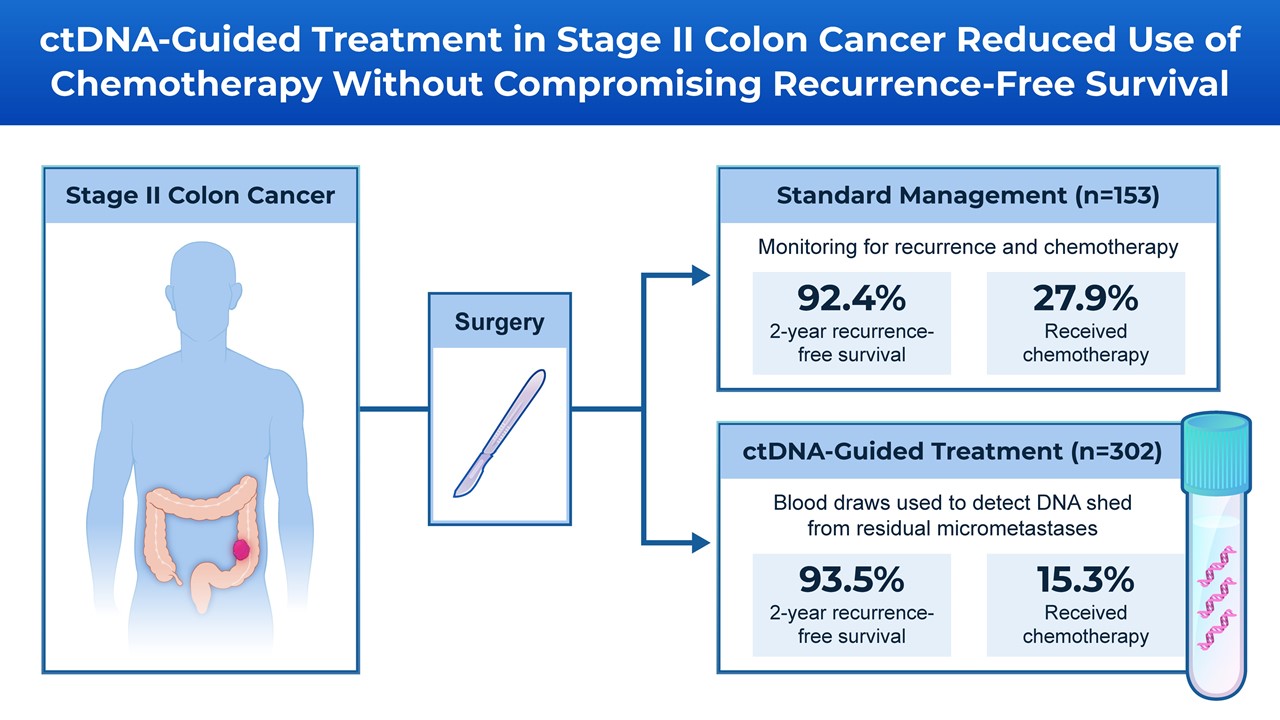
The multi-institutional, international study, led by researchers at the Johns Hopkins Kimmel Cancer Center and WEHI in Melbourne, Australia, found that testing for ctDNA after surgery and directing chemotherapy to ctDNA-positive patients reduced the use of chemotherapy overall without compromising recurrence-free survival.
There are several prior research studies demonstrating that circulating tumor DNA can be detected in blood and that the presence of ctDNA post-surgery predicts a risk of cancer recurrence. However, this is believed to be the first clinical study showing that the measurement of circulating tumor DNA prior to therapy may benefit patients.
These findings will be published in the New England Journal of Medicine and presented at the annual meeting of the American Society of Clinical Oncology on June 4.
“Previous studies have theorized that ctDNA measurements might be useful in guiding patient management, and this study provides real-world clinical evidence that supports these theories,” says Bert Vogelstein, M.D., Clayton Professor of Oncology, co-director of the Ludwig Center at Johns Hopkins and a Howard Hughes Medical Institute investigator. Vogelstein and group were the first to show that colon cancer is caused by a sequence of genetic mutations and showed that DNA shed from tumors could be detected in blood, stool and other body fluids.
Currently, the use of chemotherapy in stage II colon cancer, which is defined as a colon cancer that has grown through the wall of the colon but does not extend to the lymph nodes or other organs, is controversial. There is no consensus among cancer experts on its benefit. This study was aimed at helping solve the controversy by assessing whether ctDNA could be used to provide a more precise prediction of recurrence risk after surgery. Patients who were ctDNA-negative could be spared the toxicities of chemotherapy, and those who had remaining cancer could receive chemotherapy to attack the lingering malignant cells.
In the study, 455 patients with stage II colon cancer were randomized after surgery 2:1 to standard treatment or ctDNA-guided management. Of these patients, 153 received standard management, which includes monitoring over time for recurrence or chemotherapy. An additional 302 patients underwent blood tests within seven weeks after surgery to search for ctDNA. If ctDNA was detected, patients received fluoropyrimidine or oxaliplatin-based chemotherapy. If ctDNA was not detected, patients did not receive chemotherapy.
The ctDNA-guided approach reduced the use of chemotherapy compared with the standard management group (15.3% of patients in the ctDNA-guided group received chemotherapy versus 27.9% in the standard management group). The two- and three-year survival with no cancer recurrence was similar between the ctDNA-guided group and the standard management group.
“Stage II colon cancer presents a unique challenge,” explains Anne Marie Lennon, M.B.B.Ch., Ph.D., professor of medicine, and director of the division of gastroenterology and hepatology. “In stage I colon cancer, patients do not receive chemotherapy because their prognosis for survival is over 90%. The risk of discomfort and toxicities from the therapy outweigh the benefits it can provide. On the other hand, every stage III colon cancer patient currently receives chemotherapy because the risk of relapse is high.”
The goal of chemotherapy in colon cancer is to eradicate micrometastases, cancer cells not yet visible on radiologic images that travel through the bloodstream and cause the cancer to come back or spread it to other parts of the body. Using ctDNA to detect these invisible cells can now identify which patients are most likely to have micrometastases and, therefore, are most likely to benefit from chemotherapy.
“Using ctDNA to guide treatment, a stage II colon cancer patient who is negative for ctDNA has a lower chance of cancer recurrence than the average stage I colon cancer patient, so we have an opportunity to change clinical practice,” says Joshua Cohen, a lead author of the study and M.D./Ph.D. candidate at the Johns Hopkins University School of Medicine
The researchers hope these findings will stimulate the study of ctDNA in patients with other stages of colon cancer and other types of cancer. In future studies, the researchers will explore patients with early-stage pancreatic cancer and stage III colon cancer to see if ctDNA can similarly identify patients who are most likely to benefit from more aggressive chemotherapy than is currently used. They also plan to explore whether the presence of residual ctDNA can be used to help optimize the management of individual patients following surgery or other forms of therapy.
Using ctDNA to stratify treatments among patients is part of the movement toward precision medicine — individualized care that tailors therapies to the unique characteristics of a cancer.
The researchers also believe the findings will provide opportunities to test promising new drugs in patients with earlier stages of cancer.
“All drugs work better in patients with cancers that are detected relatively early, before they have given rise to large metastatic masses. However, new drugs are usually first tested in patients whose cancers are very advanced,” says Vogelstein. “We hope that ctDNA analysis will enable testing of new drugs in patients with early-stage cancers and micrometastases, when the new drugs are most likely to save lives.”
In addition to Vogelstein, Cohen, Lennon, other researchers were Kamel Lahouel, Ph.D., Yuxuan Wang, M.D., Ph.D., Janine Ptak, M.S., Natalie Silliman, B.S., Lisa Dobbyn, B.A., Maria Popoli, M.S., Ralph Hruban, M.D., Nicholas Papadopoulos, Ph.D., Kenneth Kinzler, Ph.D., and Cristian Tomasetti from Johns Hopkins, and Jeanne Tie, M.D., Serigne Lo, Ph.D., Suzanne Kosmider, M.B.B.S., Jeremy Shapiro, M.B.B.S., Margaret Lee, M.B.B.S., Sam Harris, M.B.B.S., Adnan Khattak, M.B.B.S., Matthew Burge, M.B.B.S. Marion Harris, M.B.B.S., James Lynam, M.B.B.S., Louise Nott, M.B.B.S., Fiona Day, Ph.D., Theresa Hayes, M.B.B.S., Sue-Anne McLachlan, M.B.B.S., Belinda Lee, M.B.B.S., and Peter Gibbs, M.D., from the Walter and Eliza Hall Institute of Medical Research, Peter MacCallum Cancer Centre, or University of Melbourne in Melbourne, Australia.
This research was supported by the Australian National Health and Medical Research Council, the Marcus Foundation, the Virginia and D.K. Ludwig Fund for Cancer Research, Lustgarten Foundation, the Conrad R. Hilton Foundation, the Sol Goldman Charitable Trust, John Templeton Foundation, National Institutes of Health (CA62924, CA009071, GM136577, CA06973) and the Eastern Health Research Foundation Linda Williams Memorial Grant.
Bert Vogelstein, Kenneth Kinzler and Nickolas Papadopoulos are founders of and hold equity in Thrive Earlier Detection, an Exact Sciences Company. Kenneth Kinzler and Nickolas Papadopoulos are consultants to Thrive Earlier Detection, an Exact Sciences Company. Bert Vogelstein, Kenneth Kinzler, Nickolas Papadopoulos and Joshua Cohen are consultants to and own equity in Haystack Oncology. Nickolas Papadopoulos and Kenneth Kinzler are on the board of directors of Haystack Oncology. The companies named above have licensed previously described technologies related to the work described in this paper from The Johns Hopkins University. Bert Vogelstein, Kenneth Kinzler, Nickolas Papadopoulos and Joshua Cohen are inventors on some of these technologies. Licenses to these technologies are or will be associated with royalty payments to the inventors as well as to The Johns Hopkins University. The university may also be entitled to equity under these agreements. This arrangement has been reviewed and approved by The Johns Hopkins University in accordance with its conflict of interest policies.


