Using computational tools, researchers from the Johns Hopkins Kimmel Cancer Center and the Johns Hopkins University School of Medicine have developed a method to assess which patients with metastatic triple-negative breast cancer could benefit from immunotherapy. The work by computational scientists and clinicians was published Oct. 28 in the Proceedings of the National Academy of Sciences.
Immunotherapy is used to try to boost the body’s own immune system to attack cancer cells. However, only some patients respond to treatment, explains lead study author Theinmozhi Arulraj, Ph.D., a postdoctoral fellow at Johns Hopkins: “It’s really important that we identify those patients for whom it will work, because the toxicity of these treatments is high.”
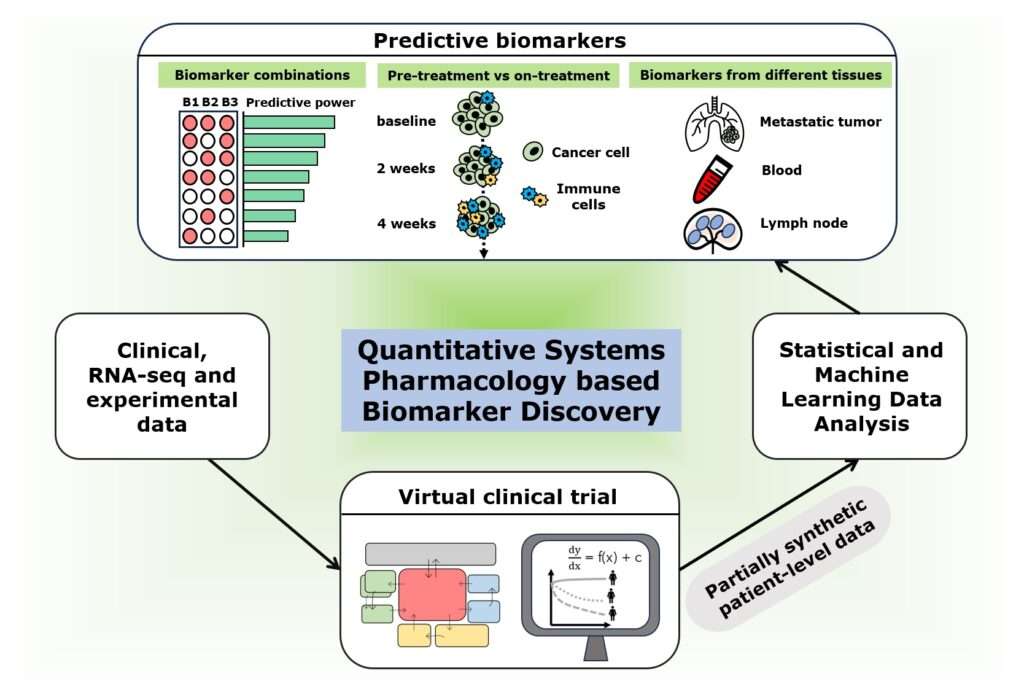
To tease this out, studies have tested whether the presence or absence of certain cells, or the expression of various molecules in the tumor, can indicate if a particular patient will respond to immunotherapy. Such molecules are called predictive biomarkers and are useful in selecting the right treatment for patients, explains senior study author Aleksander Popel, Ph.D., a professor of biomedical engineering and oncology at the Johns Hopkins University School of Medicine.
“Unfortunately, existing predictive biomarkers have limited accuracy in identifying patients who will benefit from immunotherapy,” Popel says. “Moreover, a large-scale assessment of characteristics that predict treatment response would require the collection of tumor biopsies and blood samples from many patients and would involve performing several assays, which is very challenging.”
So, the team employed a mathematical model called quantitative systems pharmacology to generate 1,635 virtual patients with metastatic, triple-negative breast cancer and performed treatment simulations with the immunotherapy drug pembrolizumab. They then fed these data into powerful computational tools, including statistical and machine learning-based approaches, to look for biomarkers that accurately predict the treatment response. They focused on identifying which patients would and would not respond to treatment.
Using the partially synthetic data produced by the virtual clinical trial, researchers assessed the performance of 90 biomarkers alone and in double, triple and quadruple combinations. They found that measurements from tumor biopsies or blood samples taken before the start of treatment, called pretreatment biomarkers, had limited ability to predict treatment outcomes. However, measurements from patients taken after the start of treatment, called on-treatment biomarkers, were better predictive of outcomes. Surprisingly, they also found that some commonly used biomarker measurements, such as the expression of a molecule called PD-L1 and the presence of lymphocytes in the tumor, performed better when assessed before the start of treatment than after treatment initiation.
The researchers also looked at the accuracy of measurements that do not require invasive biopsies, such as immune cell counts in the blood, in predicting treatment outcomes, finding that some blood-based biomarkers performed comparably to tumor- or lymph node-based biomarkers in identifying a subset of patients who respond to treatment. This potentially suggests a less-invasive way to predict response.
Measurements of changes in tumor diameter can be readily obtained by CT scans, and also could prove predictive, Popel says: “This, measured very early within two weeks of treatment initiation, had a great potential to identify who would respond if the treatment were continued.”
To validate the findings, investigators performed a virtual clinical trial with patients selected based on change in tumor diameter at two weeks after the start of treatment. “The simulated response rates increased more than two-fold — from 11% to 25% — which is quite remarkable,” Arulraj says. “This emphasizes the potential for noninvasive biomarkers as an alternative, in cases where collecting tumor biopsy samples is not feasible.”
“Predictive biomarkers are critical as we develop optimized strategies for triple-negative breast cancer, so as to avoid overtreatment in patients expected to do well without immunotherapy, and undertreatment in those who do not respond well to immunotherapy,” adds study co-author Cesar Santa-Maria, M.D., an associate professor of oncology and breast medical oncologist at the Johns Hopkins Kimmel Cancer Center with expertise in breast cancer immunotherapy and immune biomarkers. “The complexities of the tumor microenvironment make biomarker discovery in the clinic challenging, but technologies leveraging in-silico [computer-based] modeling have the potential to capture such complexities and aid in patient selection for therapy.”
Collectively, these new findings shed light on how to better select patients with metastatic breast cancer for immunotherapy. The researchers say these findings are expected to help design future clinical studies, and this method could be replicated in other cancer types.
Previously, the team used an in-house modeling framework and developed a computational model with a special focus on late-stage breast cancer where the tumor has already spread to various parts of the body. This was published in Science Advances last year. The team employed data from several clinical and experimental studies to develop and thoroughly validate this computational model.
The current work was supported by the National Institutes of Health (grant R01CA138264). Part of the work was carried out at the Advanced Research Computing at Hopkins core facility, which is supported by the National Science Foundation under grant OAC1920103.
Study co-authors are Hanwen Wang, Atul Deshpande, Ravi Varadhan, Elizabeth Jaffee and Elana Fertig of Johns Hopkins; and Leisha Emens of Kaiser Permanente in South Sacramento, California.
Popel is a consultant to Incyte and to J&J/Janssen, and is a co-founder and consultant to AsclepiX Therapeutics. He also receives research funding from Merck. The terms of these arrangements are being managed by The Johns Hopkins University in accordance with its conflict-of-interest policies.
Media Contacts
Amy Mone
Valerie Mehl
