Using artificial intelligence technology to identify patterns of DNA fragments associated with lung cancer, researchers from the Johns Hopkins Kimmel Cancer Center and other institutions have developed and validated a liquid biopsy that may help identify lung cancer earlier.
In a prospective study published June 3 in Cancer Discovery, the team demonstrated that artificial intelligence technology could identify people more likely to have lung cancer based on DNA fragment patterns in the blood. The study enrolled about 1,000 participants with and without cancer who met the criteria for traditional lung cancer screening with low-dose computed tomography (CT). Individuals were recruited to participate at 47 centers in 23 U.S. states. By helping to identify patients most at risk and who would benefit from follow-up CT screening, this new blood test could potentially boost lung cancer screening and reduce death rates, according to computer modeling by the team.
“We have a simple blood test that could be done in a doctor’s office that would tell patients whether they have potential signs of lung cancer and should get a follow-up CT scan,” says the study’s corresponding author, Victor E. Velculescu, M.D., Ph.D., professor of oncology and co-director of the Cancer Genetics and Epigenetics program at the Johns Hopkins Kimmel Cancer Center.
Lung cancer is the deadliest cancer in the United States, according to the National Cancer Institute, and worldwide, according to the World Health Organization. Yearly screening with CT scans in high-risk patients can help detect lung cancers early, when they are most treatable, and help avert lung cancer deaths. Screening is recommended by the U.S. Preventive Services Task Force for 15 million people nationally who are between ages 50 and 80 and have a smoking history, yet only about 6%–10% of eligible individuals are screened each year. People may be reticent to follow through on screening, Velculescu explains, due to the time it takes to arrange and go to an appointment, and the low doses of radiation they are exposed to from the scan.
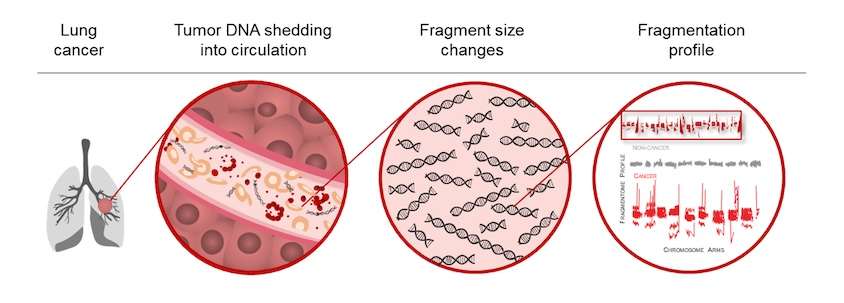
To help overcome some of these hurdles, Velculescu and his colleagues developed a test over the past five years that uses artificial intelligence to detect patterns of DNA fragments found in patients with lung cancer. It takes advantage of differences in how DNA is packaged in normal and cancer cells. DNA is neatly and consistently folded up in healthy cells, almost like a rolled-up ball of yarn, but DNA in cancer cells is more disorganized. When both types of cells die, fragments of DNA end up in the blood. The DNA fragments in patients with cancer tend to be more chaotic and irregular than the DNA fragments found in individuals who do not have cancer.
The team trained artificial intelligence software to identify the specific patterns of DNA fragments seen in the blood of 576 people with or without lung cancer. Then, they verified that the method worked in a second group of 382 people with and without cancer. Based on their analyses, the test has a negative predictive value of 99.8%, meaning that only 2 in 1,000 individuals tested may be missed and have lung cancer.
The group’s computer simulations showed that if the test boosted the rate of lung cancer screening to 50% within five years, it could quadruple the number of lung cancers detected and increase the proportion of cancers detected early — when they are most treatable — by about 10%. That could prevent about 14,000 cancer deaths over five years.
“The test is inexpensive and could be done at a very large scale,” Velculescu says. “We believe it will make lung cancer screening more accessible and help many more people get screened. This will lead to more cancers being detected and treated early.”
The test is currently available through DELFI Diagnostics for use as a laboratory-based test under the Clinical Laboratory Improvement Amendments. However, the team plans to seek approval from the U.S. Food and Drug Administration for lung cancer screening. Velculescu colleagues also plan to study whether a similar approach could be used to detect other types of cancer.
Robert B. Scharpf of Johns Hopkins co-authored the study. Additional co-authors were from the Cleveland Clinic, DELFI Diagnostics, Medicus Economics LLC, Miami Cancer Institute, the Pan American Center for Oncology, Washington University, Centura Health, Vanderbilt Health, Stratevi, Massachusetts General Hospital, the Medical University of South Carolina, the Department of Veterans Affairs, the Perelman School of Medicine at the University of Pennsylvania, New York University Langone Health, Allegheny Health Network and Memorial Sloan Kettering Cancer Center.
The work was supported in part by DELFI Diagnostics, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, Stand Up To Cancer-LUNGevity-American Lung Association Lung Cancer Interception Dream Team Translational Research Grant, Stand Up To Cancer-DCS International Translational Cancer Research Dream Team Grant, the Gray Foundation, The Honorable Tina Brozman Foundation, the Commonwealth Foundation, the Cole Foundation and the National Institutes of Health.
Velculescu and Scharpf are inventors on patent applications submitted by The Johns Hopkins University related to cell-free DNA for cancer detection that have been licensed to DELFI Diagnostics, LabCorp, Qiagen, Sysmex, Agios, Genzyme, Esoterix, Ventana and ManaT Bio. Velculescu divested his equity in Personal Genome Diagnostics (PGDx) to LabCorp in February 2022. Velculescu is a founder of DELFI Diagnostics, serves on the board of directors, and owns DELFI Diagnostics stock. Scharpf is a founder and consultant of DELFI Diagnostics and owns DELFI Diagnostics stock. Velculescu, Scharpf and Johns Hopkins receive royalties and fees from the company. The Johns Hopkins University also owns equity in DELFI Diagnostics. Velculescu is an adviser to Viron Therapeutics and Epitope. These relationships are managed by Johns Hopkins in accordance with its conflict-of-interest policies.
Media Contacts
Amy Mone
[email protected]
Valerie Mehl
[email protected]
