TriNetX is a self-service web-based data exploration tool which helps clinical researchers to define a patient cohort using inclusion and exclusion criteria and to explore cohort attributes.
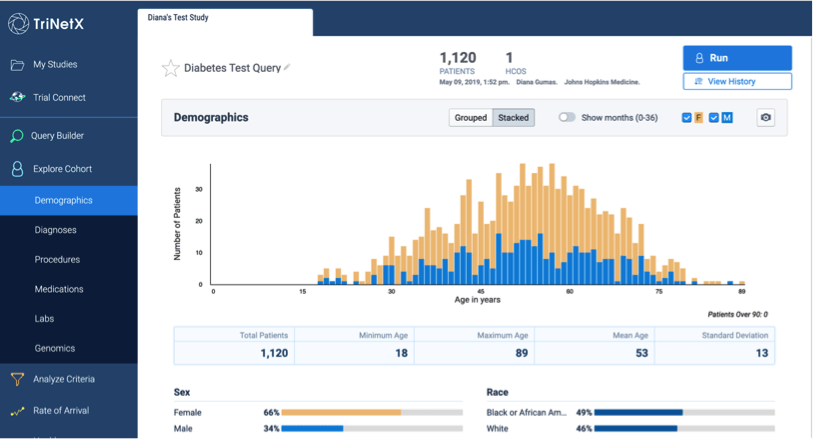 TriNetX is populated with data from Epic which have been cleaned, curated and more fully mapped to codes like LOINC to improve data quality and analysis. The date range of data in TriNetX is from July 2016 – present in order to reduce the problem of unexpected gaps in the data prior to the Johns Hopkins Medicine Epic enterprise go-live.
Some investigators may already be using Epic SlicerDicer to explore patient cohorts. While similar in concept, TriNetX and SlicerDicer have different strengths and weaknesses.
TriNetX is populated with data from Epic which have been cleaned, curated and more fully mapped to codes like LOINC to improve data quality and analysis. The date range of data in TriNetX is from July 2016 – present in order to reduce the problem of unexpected gaps in the data prior to the Johns Hopkins Medicine Epic enterprise go-live.
Some investigators may already be using Epic SlicerDicer to explore patient cohorts. While similar in concept, TriNetX and SlicerDicer have different strengths and weaknesses. TRINETX | SLICERDICER | |
Who can access? | Any staff, faculty member, or student with the JHED ID and password | Anyone with an Epic Hyperspace account with reporting privileges |
| Date Range of the Data | July 1, 2016 to present | April 2013 to present, with some legacy data prior to 2013 |
| Frequency of Updates | Bi-weekly | Nightly |
| Quality of Data | Better than what is collected in Epic | As collected in Epic |
| Types of Data | Demographics, Diagnoses, Procedures, Medications, Labs, Visits | Wider variety of data, including Family History, Providers, Epic Registries, Research Studies, Risk/Care Scores, Social History, etc. |
| Analysis Tools | Number of patients in a cohort. Exploration of that cohort’s demographics, co-morbidities, procedures, medications, and labs. Clinical phenotype criteria analysis. Rate of arrival prediction. | Number of patients in a cohort trended over time and/or stratified by subcohorts |
| Ability to Access Identifiable Data | Yes, with assistance from the Core for Clinical Research Data Acquisition (CCDA) | Physicians may access identifiable data for patients they have treated. Otherwise, help is needed from the Core for Clinical Research Data Acquisition (CCDA) |
| Online Design Assistance | Yes | No |
To get access to TriNetX you must be a Johns Hopkins faculty, staff, or student and have a Hopkins JHED account.
- View all training videos. Note that this is required before you can get an account.
TriNetX- Cohort Exploration and Research Data Analytics (optional but recommended)
- Click the “Make a Request” button at the top of this page and select “New TriNetX account” under “Core for Clinical Research Data Acquisition (CCDA) Consulting Service”.
- Acknowledge completion of the video training when prompted. You will receive an account within 2-3 business days.
TriNetX offers the ability to request a patient-level data download for either Johns Hopkins patients or for patients across multiple healthcare institutions using the TriNetX Research Network.
To request a data set (medical record number and name) for Johns Hopkins patients
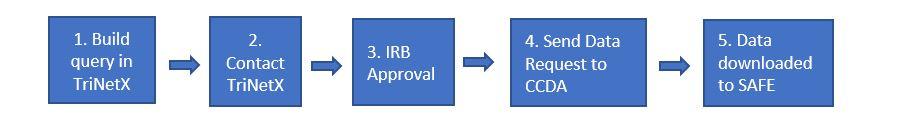
- Create a new study (query) in TriNetX. We recommend using Summary Statistics and Design Assistance features to analyze criteria and validate your cohort.
- Seek IRB approval to receive the desired data. Confirm that your query matches what was approved by the IRB or request a Change in Research to update your protocol to indicate use of the TriNetX Research Network as the data source. View a sample of the protocol language.
- Once your IRB protocol is approved, submit a request to the CCDA. Include the IRB number for your protocol in the request. The CCDA manager will schedule a brief consult with you, write a specification document, and review the IRB protocol to ensure that the query aligns with IRB approval.
- The CCDA manager will then download the patient identifiers (patient MRN, patient name) and deliver the patient list to the study team’s SAFE desktop folder.
The CCDA offers two hours of complimentary service, subsidized by the ICTR, after which an hourly rate of $118 is charged. Services include IRB review and specification development, all communications between CCDA and study team members, data download, and data delivery. Most requests can be fulfilled within the 2 hours.
- Create a new study (query) in TriNetX. We recommend using Summary Statistics and Design Assistance features to analyze criteria and validate your cohort.
- Contact TriNetX by emailing JHM’s contact, Rich Lilienthal. TriNetX will follow up with the study team to discuss the purpose of the data request and ask about study funding. For datasets requested using Research Network data which are being used for a grant, TriNetX will ask study teams to include costs for the data in the grant. Costs vary from free for most non-grant related use to high depending on use and grant funding. The investigator must negotiate the costs with TriNetX. Retain the email from TriNetX that indicates whether or not your study will need to pay TriNetX. If you will need to pay TriNetX, they should provide you with a TriNetX Dataset Order Form with negotiated cost and terms.
- Seek IRB approval to receive the desired data. Confirm that your query matches what was approved by the IRB or request a Change in Research to update your protocol to indicate use of the TriNetX Research Network as the data source. View a sample of the protocol language.
- Once your IRB protocol is approved, submit a request to the CCDA. Include the IRB number for your protocol in the request, and indicate that you are approved to receive data from the TriNetX Research Network. The CCDA manager will schedule a brief consult with you, write a specification document, and review the IRB protocol to ensure that the query aligns with IRB approval.
- The CCDA manager will submit a download request to TriNetX on behalf of the study team. TriNetX will create the dataset and make the dataset available to the CCDA. CCDA will then download the data and deliver to the study team’s SAFE desktop folder.
The CCDA offers two hours of complimentary service, subsidized by the ICTR, after which an hourly rate of $118 is charged. Services include IRB review and specification development, all communications between CCDA, study team members and TriNetX, data download, and data delivery.



