The term “clinical research” describes studies to collect new information on human health and disease.
Your doctor may have said that you are eligible for a clinical trial, or you may have seen an ad for a clinical research study. What is clinical research, and is it right for you?
Clinical research involves research volunteers to test new drugs, procedures, or devices: or to better understand how the human body works.
Every drug, device, tool, diagnostic test, technique and technology used in medicine today was once tested in volunteers who took part in clinical research studies.
At Johns Hopkins Medicine, we believe that clinical research is key to improve care for people in our community and around the world. Once you understand more about clinical research, you may appreciate why it’s important to participate — for yourself and the community.
Five Fast Facts About Clinical Research
- **The Purpose:** Clinical trials are health studies involving human volunteers. The goal is to discover answers about health and illness by testing how well a treatment works for different people. This research contributes to advancing medical knowledge and improving healthcare outcomes
- **Diversity Strengthens Research:** Representation of various backgrounds in clinical trials ensures that treatments work for everyone. Diversity makes research stronger, reflecting the uniqueness of individuals and promoting inclusivity in healthcare
- **Reasons to Join Clinical Trials:** People join clinical trials for various reasons, including interest in a health issue, compensation for time and travel, and the opportunity to be part of health discoveries
- **Safety and Compensation:** Clinical trials have multiple checkpoints to prioritize safety, and participants may receive compensation for their time or travel. This acknowledgment shows gratitude for their valuable contribution to medical research
- **Informed Participation:** Informed consent is a crucial right in clinical trials, allowing participants to know details about the study before joining. Participants can change their minds and stop participating even after the trial starts, emphasizing their control and the importance of their health
Phases of Studies
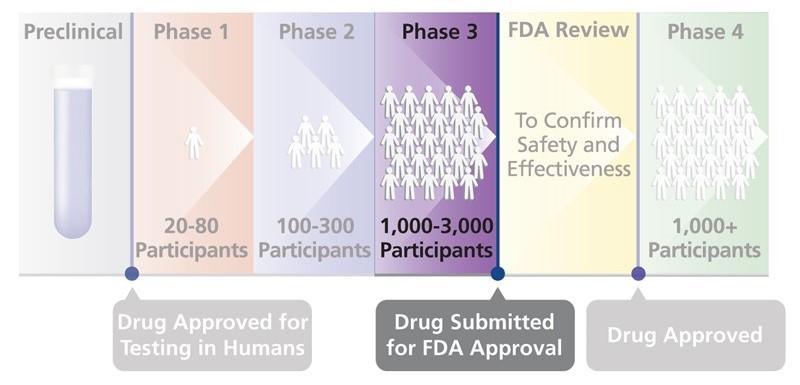
PHASE I STUDIES test a new drug for the first time in humans to see if it is safe.
PHASE II STUDIES are done in more people to see how effective the new drug is.
PHASE III STUDIES are done in large groups of people to see if the new drug works well, has side effects and how it compares to other drugs.
PHASE IV STUDIES are done after the medication is approved by the US Food and Drug Administration (FDA) to get additional information.



